eISSN: 2574-9927


Research Article Volume 6 Issue 1
Plastic Liquid Crystal Technology, Italy
Correspondence: H Hakemi, Plastic Liquid Crystal Technology, Via Lambro 80, 20846 Macherio (MB), Italy , Tel +39 349 5679838
Received: January 02, 2022 | Published: January 31, 2022
Citation: Hakemi H. The effect of a compatible liquid crystal polymer on homogeneous reinforcement of mesogenic and non-mesogenic rigid-rod monomers. Material Sci & Eng. 2022;6(1):1-4 DOI: 10.15406/mseij.2022.06.00172
Discrete amount of main-chain Liquid Crystal Polymers (LCP) could mechanically reinforce the mesophase of rigid-rod monomers to form a supramolecular homogeneous composite for Liquid Crystal Display (LCD) materials. Moreover, a homogeneously dispersed LCP may induce an enantiotropic transition in monotropic or non-mesogenic rigid-rod compounds. In this work, we studied the blends of a flexible main-chain nematic LCP with an enantiotropic nematic, a monotropic nematic, and two non-mesogenic rigid-rod compounds as model systems. The results indicate that homogenous reinforcement of thermotropic LCP in these monomers is a valid concept and could lead to improvement of mesogenic stability, orienational and mechanical properties of rigid-rod materials for various applications.
Keywords: LCP, blends, mesogenic, rigid-rod, liquid crystal, enantiotropic, monotropic
LCP, liquid crystal polymers; LCD, liquid crystal display; LCM, liquid crystal monomer
During more than five decades, the Liquid Crystal Monomer (LCM) materials have found a wide range of applications in display, optoelectronics and photonics devices. The applications typically make use of the eutectic material’s large mesogenic transition temperature and broadband optical birefringence, which as caused by molecular anisotropy combined with high sensitivity of molecular orientation to externally applied electric, magnetic, and optical fields.
Recently, heterogeneous mesogenic rigid-rod systems have attracted much scientific attention due to their additional useful properties over the conventional LCMs. By combining different constituents, it is possible to design novel smart materials with unique properties. To this end, liquid crystals have been doped with various types of additives, such as azo-dyes,1,2 ferroelectric, ferromagnetic, metallic and other nanoparticle3–7 and polymers in polymer-dispersed and polymer-stabilized liquid crystals.8,9
Doping polymers into rigid-rod monomers helps stabilize the system and at the same time provides materials with unique anisotropic properties and a limited molecular mobility in LCM systems. Adding of dye molecules to a LCM matrix usually does not change the basic matrix properties such as elasticity, clearing temperature, electro-conductivity, refractive index or the other dielectric properties, provided that the dye concentration is less than 1-2 %wt. The most significant change in this case is the appearance of an absorption band at a desired wavelength range of the spectrum.
The non-covalent interactions between molecules of different substances have also stimulated the preparation of new LCM composites. However, most publications so far related to non-covalent LCM systems are limited to the so-called functionalized thermotropic polymers, i.e., LCP matrices modified with low-molar-mass dopants, such as photochromic molecules.10
In comparison with LCM materials, the long-chain LCP materials exhibit larger bulk viscosity and superior mechanical properties. The unexplored self-supporting display devices based on mesogenic state of main-chain LCPs is a new interesting possibility, where side-chain LCPs have received particular attention in this context.11 However, the high viscosity and transition temperature of main-chain LCPs contradicts with the need for fast response times and lower temperatures typical of LCD devices. The guest-host systems in which the macroscopic domains of a LCM are heterogeneously dispersed in a matrix of non-mesogenic polymer has been considered as a way to blend the most favorable properties of the two components where cross-linked gels of a nematic LCP and a LCM has been studied.12
A homogeneous dispersion of a main-chain LCP in mesogenic rigid-rod is an unexplored strategy for “mechanical reinforcement” of these materials in LCD device applications. Since no chemical bonds stabilize the assembled composite, one expects only a minor alteration of the fast response times. Essentially the mechanical properties of such composites could be modulated by an alteration of their compositional ratio. Dispersions of solute amounts (ca. 1%) of a synthesized polymer in the presence of a LCM solvent has been already reported, but such composites did not exhibit molecular dispersion at the investigated temperatures.13
The compatibility dispersion of a main-chain LCP in a LCM is the main obstacle to the formation of a homogeneous molecular composite, particularly over a large compositional range, where both chemical and conformational compatibility are to be considered.14,15 In order to insure the chemical compatibility, it is necessary to select a low molecular weight LCP with similar chemical structure to provide molecular recognition with LCM component.
A useful thermodynamic criterion for assessing the chemical compatibility is the comparison of Flory’s solubility parameter with the corresponding critical values cc at which de-mixing occurs.16 Also in order to insure the conformational compatibility, the shape recognition must occur in which both LCP and LCM components exhibit adequate molecular similarity and asymmetry.15 It is known that, the blends of low or high molecular weight mesogens with coiling polymer chain do not fulfill the conformational compatibility and de-mixing occurs even at a small (<1%) polymer volume fraction for molecular weights larger than about 2000.17,18 In our previous work, we have shown that a liquid crystal polyester based on the rigid segment 4’-hydroxyphenyl-4-hydroxybenzoate with methylene spacer to exhibit good compatibility within the whole compositional range of blends with a LCP having the same rigid unit, but shorter flexible sequence.18
In the present work, we propose the new concept of homogeneous reinforcement of mesogenic and non-mesogenic rigid-rod monomers by a compatible LCP material, where we utilized a semi-flexible and low molecular weight LCP model and studied its phase diagram with three types of rigid-rod monomers, including an enatiomeric nematic, a monotropic nematic and two non-mesogenic compounds. Although the utilized materials were not ideal, the results of these preliminary model composites indicate that a homogeneous reinforcement is a viable approach to improve the mesomorphic, self-orienting and mechanical properties of rigid-rod monomers for a wide range of potential applications.
The LCP model material designated as S7was a flexible homo-polyester based on 4’ – hydroxyphenyl 1-4-hydroxyenzoate rigid backbone and a flexible [- (CH2)n -] moiety with n=7 with the following repeat unit chemical formula:
The synthetic procedure of S7 polymer has been reported elsewhere and has a distinct characteristic of low molecular weight LCP with stable enantiotropic nematic phase.19,20 The four rigid-rod model materals utilized in this study were an enantiotropic (MT), a monotropic (DA) and two non-mesogenic (DO, TP) materials with the following chemical structures:
The enantiotropic MT monomer was obtained from Eastman Kodak. The monotropic DA and non-mesogenic DO monomers were the intermediate products of S7 synthesis reported earlier.21 The non-mesogenic TP compound was obtained from Aldrich Chemical and was used without further purification.
The average asymmetry of the molecular structures of monomers and polymer repeat unit were determined on a molecular model at extended trans configurations. The densities of pure components were determined by floating technique at ambient temperature.
The thermal properties and physical parameters of S7 polymer and four rigid-rod monomers are tabulated in Table 1. According to transition temperature data of Table 1, both S7 and MT are enantiotropic nematic, DA is a monotropic nematic, whereas DO and TP are non-mesogenic which directly melt into isotropic phase and solidify into crystalline state by heating and cooling, respectively.
|
|
TCI (°K) |
TCN (°K) |
TNI (°K) |
Enthalpy |
Entropy |
Axial Ratio (x) |
Density |
|
Compound |
(KJ/mole) |
(J/K.mole) |
(g/ml) |
||||
|
S7 |
- |
466 |
520 |
C (10.6) N (4.2) I |
C (22.7) N (8.1) I |
3.70** |
1.24 |
|
MT |
- |
460 |
608 |
C (48.7) N (6.5) I |
C (106) N (10.7) I |
5 |
1.21 |
|
DA |
438 |
- |
(396)* |
C (48.6) I |
C (111) I |
3.3 |
0.66 |
|
DO |
519 |
- |
- |
C (48.7) I |
C (93.8) I |
2.45 |
1.2 |
|
TP |
487 |
- |
- |
C (37.7) I |
C (77.4) I |
2.55 |
1.01 |
Table 1 Thermal properties and physical parameters of S7 polymer and rigid-rod monomers
*On cooling, **Polymer repeat unit
The transition temperatures and the corresponding enthalpies and entropies of S7 /rigid-rod monomer blends were obtained by Dupont model 910 DSC Differential Scanning Calorimetry (DSC) and Olympus model BH-2 polarizing optical microscope (OM) equipped with Mettler FP52 hot-stage microscope and FP5 temperature control unit. The blends were prepared by direct weighing and mechanical mixing of the components in DSC pans above nematic-isotropic temperatures. In order to achieve optimum blending, the DSC thermograms were taken on repeated heating/cooling cycles until they were reproducible. The OM measurements were subsequently performed on these DSC samples.
In Figure 1, we present the transition temperatures of S7/MT, S7/DA, S7/DO and S7/TP blends within the total phase diagrams as a function of S7 volume fraction by both DSC and OM methods. Although these blends exhibited biphasic regions in all transition temperatures,22,23 but for simplicity, here we only plotted the mean-values of transition temperatures. The interaction parameter c calculated from the four phase diagrams were smaller than their critical parameter cc, which confirms their chemical compatibility.15
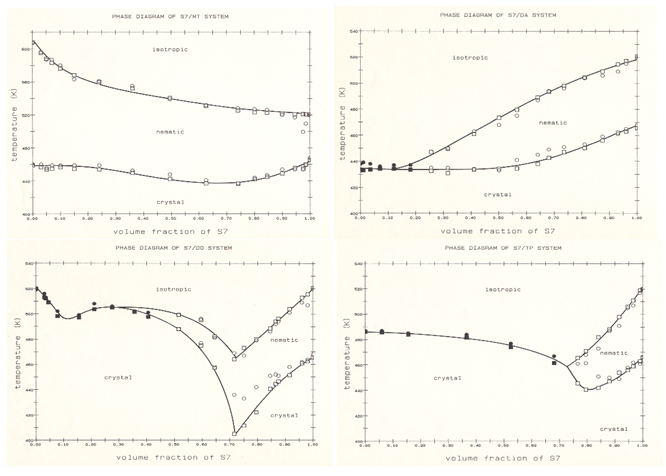
Figure 1 Phase diagrams of S7/MT, S7/DA, S7/DO and S7/TP measured by DSC (circles) and OM (squares) techniques. The filled symbols are those of TCI transitions.
With regards to S7/MT phase diagram, both chemical and conformational compatibility conditions prevail where a single mixed nematic phase occurs over the whole composition range. Also an ideal mixing behavior is exhibited in TNI transitions of S7 and MT components (Table 1). On the other hand, the TCN transitions are depressed by either compound and a mild eutectic behavior is evident at around 70% of S7 volume fraction. Similar type of compatibility between segmented polyesters and mesogenic rigid-rod materials have been also reported by others.24,25
Also regardless of biphasic spread in S7/MT phase diagram, the overall linear trend of TNI confirms the asymmetric correlation, chemical and conformational compatibility due to homogeneous miscibility of S7 and MT components, similar to rigid and semi-flexible main-chain LCP blends reported elsewhere.26,27 Although in S7/MT model system the nematic stability of MT is almost twice wider than that of S7, but at small comopsitions (< 1%) of S7, the nematic stability range of MT is minimally reduced. Instead, the presence of S7 provides a homogeneous reinforcement of the blend by enhancing its mechanical and orientational properties, as well as suppression of crystal structure of MT.28 This is a relevant proof for improving the properties of a typical LMC by dopping of a properly designed main-chain LCP for application in commercial LCD devices.
The phase transitions of S7/DA blends (Figure 1) is also interesting and requires explanation. As it has been mentioned before, DA is an intermediate of S7 sythesis with similar molecular structure to S7 repeat unit and exhibits an inherent monotropic nematic phase. The nematic stability of S7/DA over the phase diagram is too large to be attributed to a segmented chain dilution by conventional diluent.15 Due to the similarity of DA and S7 molecular structures, it would be unlikely that the large nematic stability of S7/DA blends is the result of transesterification reaction. Although, the transesterification reaction has been previously reorted in heat-treated polymer blends with higher homologue of DA.25 However in that case, due to high transition temperature of smectic material, the heat-treatment of blends had been carried out at much higher temperature which had resulted to transesterification. Alternatively, at above >0.2% volume fraction the S7 inhances the enantiotropic state of DA and by increasing the S7 composition, the nematic phase of blends expand at the expense of suppressing the crystalline structure and monotropicity of DA. Interestingly, by extrapolation of TNI transition curve to zero composition of S7, it intersects with the temperature axis at around 400K which is near the monotropic transition temperature of DA at TNI = 396K (Table 1). Consequently, in the case of S7/DA composite system, it is expected that additional reinforcing effect and stabilization of mesophase should be evident with other types of monotropic rigid-rod structures.
With respect to S7/DO phase diagram,we found that its nematic phase is more extended and its TNI eutectic behavior is more distinct than those in S7/TP phase diagram (Figure 1). This difference cannot be only argued on the basis of their molecular asymmetry argument. At present, the only speculative conclusion for such unexpected result could be due to active hydroxyl end groups of DO and its interactions with S7 that stabilizes the nematic phase. This effect becomes insignificant at the lower composition range of S7 (< 0.5) and the contribution of DO in the phase equilibria of S7/DO system should predominantly be due to the steric effect.
In the case of S7/TP blends (Figure 1), although TP is a conventional non-mesogenic rigid-rod material, the behavior of their phase diagram is also expected, where the blends of TP as solute and S7 polymer as solvent exhibit a nematic phase at high concentration range of S7. It should be emphasized that, as the chemical and conformational compatibilities in S7/TP blends occur at high composition range of S7, this system cannot be considered an appropriate model system presenting the concept of homogeneous reinforcement proposed in this study.
We presented the new concept of homogeneous reinforcement of mesogenic and non-mesogenic rigid-rod materials by addition of a compatible main-chain LCP solute to enhance their mesogenic, orientational and mechanical properties.
The studies of mesogenic phase of S7/MT and S7/DA model systems present cases in which the mesophase of a single (or eutectic) rigid-rod compounds could admit a modest amount of a compatible LCP solute. The phase diagrams of such binary blends indicate a complete mesophase miscibility of S7 within the whole MT/S7 phase diagram. Such miscibility occurs at >20% in S7/DA, >50% in S7/DO and >70% in S7/TP phase diagrams. Accordingly, in both mesogenic and non-mesogenic monomers the mesomorphic stability is directly proportional to the similarity of molecular structure and compatibility between LCP repeat unit and rigid-rod structure.
Although the presented LCP/rigid-rod models are not ideal, the results suggests enhancement of mesogenic miscibility, chemical and conformational compatibility of the components. For the purpose of application, the liquid crystal and mechanical properties of eutectic LCM material requires improvement through molecular engineering and synthesis of new compatible LCP structures. Eventually, low doping of eutectic LCM compounds with compatible mesogenic LCP could further improve the transition temperature and supress the crystal-nematic transition temperatures of LCM and avoid the LCP gelation.
The author would like to extend his gratitude to late Professor W.R. Krighbaum (Duke University) and late Professor A. Ciferri (University of Genova), who provided the author to carry out the experimental parts and proposed the basic concept and of this study during 1983-84 academic period, respectively.
The author state that there is no conflict of interest.
None.

©2022 Hakemi. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.