eISSN: 2574-9927


Research Article Volume 7 Issue 1
1Mineral Processing Engineering Department, Istanbul Technical University, Turkey
2Julius Kruttschnitt Mineral Research Center, Sustainable Minerals Institute, The University of Queensland, Australia
3Metallurgical & Materials Engineering Department, Istanbul Technical University, Turkey
Correspondence: Fatma Arslan, Mineral Processing Engineering Department, Istanbul Technical University, Maslak 34469, Istanbul, Turkey, Tel 90 212 285 6357
Received: December 26, 2022 | Published: January 19, 2023
Citation: Arslan F, Güven ZB, Arslan UY, et al. Solvent extraction of nickel from iron and cobalt containing sulfate solutions. Material Sci & Eng. 2023;7(1):1-6. DOI: 10.15406/mseij.2023.07.00195
In this study, solvent extraction is applied for extracting nickel from sulfate solutions containing iron and cobalt ions. This process can also be applied for the cleaning of waste solutions and for increasing solution concentrations after the leaching of low-grade nickel ores. Di-2-Ethylhexyl phosphoric acid (D2EHPA) was used as an extractant for this purpose. Effects of pH, aqueous/organic (A/O) phase ratio, iron, and cobalt ion contents of the solution on nickel extraction efficiency were investigated. The McCabe-Thiele diagrams for extraction and stripping steps were developed to determine the number of stages required. A total of two stages were required for 96.3% of Ni extraction and a total of two stages to strip nickel with 98% efficiency.
Keywords: Nickel; iron; cobalt; D2EHPA; solvent extraction
The presence of heavy metals in waste streams may result in many environmental problems and sometimes the deterioration of numerous ecosystems. The accumulation of such polluting ions is due to improper management of resources and increased industrial activities. Therefore removal of these ions is necessary and must be carried out because of the environmental and economical viewpoints. The wastewater streams are fractionated into two or more liquid phases that are either process recyclable, saleable, or waste.1 Solvent extraction (SX), membrane-based process adsorption, and ion exchange technology are the main separation processes for these purposes. SX is one of the most efficient methods used to remove, separate, and concentrate metallic ions from coexistent species such as Zn, Co, Ni, Cu, etc. The use of liquid-liquid extraction technology in the industry has increased rapidly since 1970, and in the last 15 years, there have been incremental advances in the application of SX in hydrometallurgy.2 SX in hydrometallurgical processes is used to separate the various metals and numerous studies have been carried out on this technology for the purification of solutions obtained by processing ores and waste treatment, such as spent catalysts, spent batteries, and other waste solutions.
In hydrometallurgical processes, solvent extraction (SX) is a well-established technique for the separation of various metal ions after the leaching of low-grade ores and their removal from waste solutions. In fact, the technique can be applied to the treatment of both concentrated and dilute solutions. Solvent extraction is a unit operation for the purification and concentration of a wide variety of metals. Solvent extraction is a common form of chemical extraction using organic solvent as the extractant. It is commonly used in combination with other technologies, such as solidification/stabilization, precipitation, and electrowinning. It is able to produce pure metal solutions which are used for electrowinning purposes.3 Many metal separations are being done commercially by extraction of aqueous solutions, such as Cu, Co, Zn, U, Mo, W, V, Zr, Hf, Nb, Ta, Ga, Ge, rare earth, and the platinum group metals (PGMs). In order to recover the extracted metal species from the organic phase as well as for recycling purposes of the reagent, the metal species in the organic phase is stripped with relatively strong acids such as H2SO4 or HCl. During the extraction process, a hydrogen ion on the organic reagent is replaced with the metal species in the aqueous phase, but during the stripping process, the hydrogen ion from the stripping agent (H2SO4 or HCl) replaces the metal species on the organic reagent. Hence during the stripping process, the organic reagent is regenerated to its acidic form and recycled to serve as extracting reagent while the metal species is recovered for further processing. A schematic illustration of a typical solvent extraction circuit is shown in Figure 1.3
Primarily nickel can be obtained from the leaching of laterite ores and sulfide ores.4,5 The leach liquors obtained by the leaching of ores contain a significant amount of nickel along with copper and other metallic impurities. Due to the rapid depletion of primary resources and ever-increased demand for nickel, secondary resources have gained recent attention. These secondary resources are spent catalysts, alloy scrap, sludge, waste streams from plating plants, metal finishing industries, spent metallic batteries, and dust that can be used for cobalt/nickel production.6,7 Manganese nodules found in oceans are also considered potential future resources for nickel and cobalt.8 Sources of nickel are summarized in Figure 2.9
The separation of cobalt and nickel in an aqueous solution has always been a problem in hydrometallurgy.10,11 Their adjacent positions in the transition of metal series in the periodic table result in aqueous chemical behavior that is too similar for the development of easy separation routes. Both metals exist as divalent hexa-hydrated ions in dilute solutions. However, small differences in chemical behavior do exist such as water exchange of cobalt ions much higher than for nickel; thus complex formation often proceeds much more readily with cobalt than with nickel ions. Further, the development of alkyl phosphoric acid-based compounds such as phosphoric (D2EHPA), phosphonic (PC 88A), and phosphinic (CYANEX 272) acids revolutionized the separation of cobalt from nickel from sulfate solutions.12 Traditionally nickel and cobalt separation is based on selective oxidation and/or precipitation of cobalt from either sulfate or chloride solution and such processes are still in use today. However, the solvent extraction process provides a high degree of separation and yields demanded by industry nowadays.
The separation of cobalt and nickel by SX has been studied quite intensively over more than 25 years. Several extractants such as CYANEX 272, DEHPA, PC88A are used.10,12,13 The separation factor increases in the series of phosphoric/phosphonic/phosphinic acids. The selectivity series also undergo changes within the series of phosphoric, phosphonic, and phosphinic acids as shown below:
DEHPA Fe3+ >Zn>Ca>Cu>Mg>Co>Ni
PC88A Fe3+>Zn>Cu>Ca>Co>Mg>Ni
CYANEX 272 Fe3+>Zn>Cu>Co>Mg>Ca>Ni
The use of mixed extractants, such as mixtures of TOPS 99 & TIBPS is now gaining attention to overcome the problems that arise from utilizing single extractants.14 Synergic systems consisting of carboxylic acid and aliphatic hydroxy oxime were also studied.15 The extraction of nickel by chelating extractants, can be represented by a general equation as follows:
Niaq++ + 2HRorg ↔ NiR2 org + 2Haq +
In the hydrometallurgy of laterite nickel ores, solvent extraction (SX) has been an important separation technique to enrich the nickel and its primary by-product, cobalt. This is because SX is currently the only proven commercial technique to separate the two chemically-similar metals from the pregnant leach solution (PLS). Therefore, SX operation is preferred if separate high-purity products of nickel and cobalt are targeted.16 Because copper, cobalt, and nickel often occur together in nature and because of their industrial importance, several papers reporting their separation by solvent extraction with various extracting reagents have appeared in the literature.17
There are some studies on solvent extraction of nickel from solutions containing some other metal ions such as Fe, Cu, Zn, Co, Cr, etc. Makino et al.18 studied solvent extraction of nickel using an organic acid from a crude nickel sulfate solution containing impurities such as sodium and ammonium, using an alkyl phosphonic acid or its esters, such as CYANEX 272, D2EHPA, PC88A, or the like, as the extractant.18 In this study, the desirable pH was kept between 5 and 7 in consideration of the residual loss of nickel in the extraction residue. It was found that if the extraction is carried out at a pH higher than that of raffinate, a large amount of sodium, ammonium and other impurities may be extracted.
In an earlier study on the separation of nickel and zinc ions from synthetic solutions by SX using D2EHPA and CYANEX 272, the pH range for Ni extraction was found 4.5 and 7.5, respectively.19 The stripping study was performed using sulphuric acid and it was shown that above 98% zinc and nickel could be extracted. Nickel stripping from the loaded organic phase was studied using sulphuric acid and the stripping efficiency of nickel reached 98% using 2M sulphuric acid.
In another study, the effect of sodium acetate, sodium citrate, and the addition of sodium oxalate was investigated in the D2EHPA system for the extraction of nickel and copper.20 Furthermore, the effect of various parameters such as pH, the concentrations of carboxylates, and time was determined. By adding 1 g/L sodium oxalate to 20% D2EHPA, the maximum separation of nickel and copper at the concentrations of 500 mg/L was obtained at the pH of 5 within a time of 10 min. For the mixture system of sodium acetate and sodium oxalate at the concentrations of these agents being 2 g/L, 10 min, and optimum pH of 5.12, the maximum separation was achieved. In the presence of sodium acetate, the extraction efficiency of both nickel and copper was high, indicating that this agent has no effect on the separation of these ions.
A study on the synergistic green solvent extraction of nickel ions from real electroless nickel plating wastewater using an inorganic phase containing binary mixtures of Di(2-ethylhexyl) phosphoric acid (D2EHPA) and octanol in palm oil was conducted.21 Results revealed that both D2EHPA and octanol acted as a carrier and synergists respectively. The green binary mixtures D2EHPA-octanol system is capable of extracting the nickel from real electroless nickel plating waste water up to 90% at the best condition of 0.7M D2EHPA and 15% (v/v) octanol in palm oil. Subsequently, 1.0M nitric acid is employed as a stripping agent for the back extraction of nickel from the nickel-loaded organic phase. According to the stoichiometry study for nickel extraction, only one mole of D2EHPA and nitric acid was involved in the extraction and stripping reaction of nickel. It was also found that the green organic phase can be recycled back for nickel extraction as well as minimizing the use of an expensive carrier in the solvent extraction process.
Eyuboglu & Kumbasar22 studied to explain the synergistic extraction of nickel from simulated Cr-Ni electroplating bath solutions (SEBS) using 5,8-diethyl-7-hydroxydodecane-6-one oxime (LIX 63) and di-(2-ethylhexyl) phosphoric acid (D2EHPA) as extractants by emulsion liquid membrane (ELM) technique.22 In this experimental study some important parameters like acid concentration, stripping solution type and concentration, mixing speed, extractant concentrations, phase ratio, and surfactant concentration were studied to improve the extraction and stripping efficiencies. Higher than >99% of nickel was recovered at the optimum conditions within 6 min and the nickel extraction kinetic with D2EHPA has been defined as faster than LIX63. According to these results, it was revealed that D2EHPA behaved as a synergistic extractant in that extraction mechanism.
Soeezi23 studied the effect of the extraction of D2EHPA on the extraction of copper and nickel ions in a synthetic solution containing 770 ppm copper, 3200 ppm nickel, 800 ppm iron, and 200 ppm zinc by changing the levels of parameters such as speed and time of mixing, the concentration of extractor, acidity (pH), and temperature.23 The best and highest extraction of copper and nickel was obtained at a time of 5 min, a speed of 700 rpm, a temperature of 45°C, an extractor concentration of 30%, and an A/O ratio of 3 at pH 6.
The synergistic effect of Cyanex 302 on solvent extraction of nickel and cadmium from sulfate solution with D2EHPA diluted in kerosene was also investigated by Babakhani et al.24 Experiments were carried out within a pH range of 0.1-5.0 using sole D2EHPA and D2EHPA-Cyanex 302 mixtures in different ratios. In the presence of the sole D2EHPA, the percentage of extraction for both cadmium and nickel increased with increasing the equilibrium pH. Cd extraction with D2EHPA, however, involves co-extraction of nickel. Adding Cyanex 302 to D2EHPA caused a synergistic effect and shifted the extraction curve of cadmium and nickel to the left and right, respectively. As a result, optimum separation was found with a Cyanex 302 to D2EHPA ratio of 0.1M/0.5M, and increasing Cyanex 302 to D2EHPA ratios in the organic phase caused a left shifting of the extraction isotherm of cadmium and a right shifting of the extraction isotherm of nickel.
In this experimental study, the solvent extraction process was utilized in order to extract nickel separately from sulfate solutions containing iron and cobalt as an example of the cleaning of waste solutions and increasing solution concentrations after leaching. Experiments were carried out at varying pH, Aqueous/Organic (A/O) ratio, and nickel/cobalt/iron concentrations. These findings will give important information on the separation of impurities from waste solutions and the recovery of metals from leach liquors produced as a result of the dissolution of low-grade nickel ores containing iron and cobalt minerals.
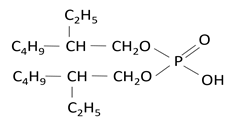
A solvent extraction process was developed to recover nickel using 10 wt% of di-2-ethylhexyl phosphoric acid (D2EHPA) as an extractant (Table 1).25 Synthetic solution containing 1 g/L Ni and/or 1 g/L Fe and 0.5 g/L Co was prepared by dissolving required amounts of NiSO4•6H2O, FeSO4•7H2O and CoCl2•6H2O in distilled water and used in the solvent extraction studies. Kerosene was used as the diluent. Dilute sulfuric acid was used as a stripping agent for the stripping of metal ions from the loaded organic. Figure 3(a) & 3(b) shows the mixer and separating funnel used in the solvent extraction experiment adapted from the basic mixer-settler equipment and experimental flow diagram.
|
D2EHPA |
Properties |
|
|
Molecular formula |
C16H35O4P |
|
Molar mass (g/mol) |
322.43 |
|
|
Boiling point (oC) |
393 |
|
|
Physical state (room temp.) |
Liquid |
|
|
Density (g/mL) |
0.9758 |
|
|
Appearance |
Odorless yellow liquid |
Table 1 Chemical structure and properties of D2EHPA
All solvent extraction experiments were carried out by shaking equal volume (except for the McCabe-Thiele construction tests) of synthetic aqueous solution and desired extractant of known concentration in a separating funnel for 15 minutes which was found to be sufficient to reach equilibrium. The pH of the aqueous solution was adjusted to the desired value by adding dilute H2SO4 or NaOH before equilibrium. After the phase disengagement, the aqueous and organic phases were separated. Metal ion concentrations in the aqueous phase were analyzed by Atomic Adsorption Spectrophotometer (AAS). Metal contents of the organic phases were determined by mass balance.
In the solvent extraction experiments, the effects of pH, aqueous/organic (A/O) ratio, and iron and cobalt additions on nickel extraction were investigated and the results are given below in detail.
Effect of pH
The effect of pH on the extraction efficiency of nickel was investigated under the experimental conditions of 15 minutes of extraction time, 10% of volume fraction of extractant D2EHPA, A/O ratio of 1/1, and stirring speed of 500 rpm. The results are shown in Figure 4. Increasing pH values to the basic range resulted in an enormous increase in extraction efficiency. A maximum of 80% of Ni extraction efficiency was reached at the pH value of 12. Since the extractant D2EHPA was degraded after pH 12, further experiments were carried out in the pH range of 10-12. In the literature findings on SX experiments of Ni from solutions containing some other ions, pH range studied was between 1-7.5 18,19,20,23,24 where our pH range was between 1-12 and increasing pH had also an increasing effect.
Effect of Aqueous/Organic (A/O) ratio
In the experiments for investigating the A/O ratio effect, an initial Ni concentration of 1 g/L, pH range of 9-9.4, 10% of D2EHPA concentration, 500 rpm stirring speed, and 15 minutes of stirring were kept constant while the A/O ratio was varied. Results are shown in Table 2. Decreasing the A/O ratio had a decreasing effect on the nickel extraction efficiency starting from 41.3% to 36.7% with the increased organic addition. Therefore, the 1/1 A/O ratio was accepted as an optimum value.
A/O |
Ni in aqueous |
Ni in organic |
% Ni |
1/1 |
0.587 |
0.413 |
41.3 |
1/2 |
0.605 |
0.395 |
39.5 |
1/3 |
0.627 |
0.373 |
37.3 |
1/4 |
0.633 |
0.367 |
36.7 |
Table 2 Effect of A/O ratio on the solvent extraction of Ni
Effect of iron and cobalt ions in the solution
The effect of the presence of Fe and Co ions in the Ni-containing sulfate solutions was also studied and the results were summarized in Table 3. The addition of Fe ions alone had an increasing effect on Ni extraction efficiency from 48% to 61.8% while 96.2% of Fe is extracted. The addition of Co ions alone did not affect Ni extraction while 80.26% of Co was extracted resulting in the extraction of both metals at the same time. The separation of cobalt and nickel in an aqueous solution is not possible due to their adjacent positions in the transition of metal series in the periodic table resulting in too similar aqueous chemical behavior as mentioned in earlier studies.10 When Fe and Co ions are present together in the solution, all metals were extracted at the same time in a similar manner when they have added alone. Ni extraction with D2EHPA, however, involves co-extraction of iron and cobalt with small increases in the extraction of all metal ions. Decreasing the concentrations of all metal ions resulted in increases in all metal extractions as shown in Table 4.
Initial metal ion concentration |
% Ni |
% Fe |
% Co |
1 g/L Ni |
48 |
- |
- |
1 g/L Ni + 1 g/L Fe |
61.8 |
96.2 |
- |
1 g/L Ni + 0.5 g/L Co |
46.6 |
- |
80.26 |
1 g/L Ni + 1 g/L Fe + 0.5 g/L Co |
60.1 |
99.64 |
83.8 |
Table 3 Effect of presence Fe and Co ions on the extraction efficiency of nickel
Initial metal ion concentration |
% Ni |
% Fe |
% Co |
0.18 g/L Ni + 0.18 g/L Fe |
74.53 |
92.38 |
- |
0.18 g/L Ni + 0.09 g/L Co |
75.87 |
- |
75.39 |
0.18 g/L Ni + 0.18 g/L Fe+ 0.09 g/L Co |
73.35 |
99.44 |
76 |
Table 4 Effect of initial metal concentrations on the solvent extraction efficiency of nickel
Table 5 shows the stripping results after loading the metals from a solution containing 1 g/L Ni, 1 g/L Fe, and 0.5 g/L Co ions. Fe stripping efficiency is very low (0.58%) while Ni and Co striping efficiencies are 53.74% and 66.67%, respectively.
Metal ions |
Initial metal Conc. (g/L) |
Metal conc. in organic phase (g/L) |
Stripped metal conc. (g/L) |
Stripping efficiency (%) |
Ni |
1 |
0.601 |
0.323 |
53.74 |
Fe |
1 |
0.996 |
0.006 |
0.58 |
Co |
0.5 |
0.338 |
0.212 |
66.67 |
Table 5 Stripping efficiencies of Ni, Co, and Fe
During the hydrometallurgical processing of the major base metals such as Cu, Zn, Ni, and Co, the presence of Fe is normally a serious complication and iron separation from these metals constitutes one of the main challenges for the producers.26 There are many instances; however the presence of iron is beneficial, or is even required. In our case, the presence of iron in extraction had a positive effect by increasing the extraction efficiencies of Ni and Co metals. In the stripping stage, having very low Fe content in the solution is also found beneficial due to making further purification easier.
McCabe-Thiele Diagrams
In order to find out the number of stages required for the complete extraction of nickel from the sulfate solution, the McCabe-Thiele plots were evaluated for extraction and stripping steps, separately. Experimental conditions were: initial Ni concentration of 1 g/L, 10% D2EHPA, A/O phase ratio of 1/1, 500 rpm stirring speed, and 15 minutes of stirring time. Extraction isotherm shown in Table 6 and Figure 5 indicate that a total of two stages were required for 96.3% of Ni extraction with 10% D2EHPA solution using the A/O phase ratio of 1:1.
Number of loading stages |
Ni in aqueous phase |
Ni in loaded organic phase (g/l) |
Ni extraction efficiency (%) |
1 |
0.45 |
0.55 |
45 |
2 |
0.017 |
0.43 |
96.3 |
3 |
0.002 |
0.015 |
88.24 |
Table 6 Ni Extraction Results related to loading stages
Stripping studies of nickel from the loaded extractant D2EHPA were carried out by contacting the organic phases in 15 minutes with a 2M sulfuric acid solution as given in the literature.19 The results of stripping studies are given in Figure 6. According to that, it is theoretically possible to strip nickel with 98% efficiency in two stages.
Solvent extraction was applied for extracting nickel from sulfate solutions containing iron and cobalt. D2EHPA was used as an extractant and the effects of pH, aqueous/organic (A/O) phase ratio, and metal ion (Ni, Fe, Co) concentrations of the solution on nickel extraction efficiency were investigated. The following conclusions are made as a result of this experimental study:
None.
None.
There are no conflicts of interest.

©2023 Arslan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.