In this research paper, thin films of polyethersulfone reinforced with different concentrations of the zinc-nickel ferrite nanoparticles [ZnNiFe] using the spin coating technique is presented and briefly discussed. The polymer chosen was polyethersulfone. Optical characterization of the test samples was carried out using different spectroscopic techniques at room temperature [25°C]. The structural properties, texture, and morphological characteristics of the as-prepared thin films were studied using the techniques of Fourier transform infrared spectroscopy [FTIR], X-ray diffraction [XRD] spectra, atomic force microscopy [AFM], and scanning electron microscopy [SEM]. The results revealed the ZnNiFe nanoparticle-reinforced polyethersulfone thin films are good candidates for selection and use in photonic applications, such as optical sensors, solar panels, and other photonic devices. In addition, both the ground state dipole moment and excited state dipole moment were determined for the polyethersulfone (PES) molecule using the solvatochromic shift method. This was essentially for developing an understanding of the charge transfer characteristics of the solute. Results of this novel study did reveal the excited state dipole moment to be observably greater than the ground state dipole moment.
Keywords: polyethersulfone, zinc-nickel ferrite nanoparticles, spin coating technology, ultra violet (UV-Vis), Scanning electron microscopy (SEM), photoluminescence, atomic force microscopy (AFM), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD)
Photonics is all about the science of light. Photonics involves a very wide range of wavelengths ranging from gamma rays to radio waves, which includes the infrared, ultra-violet (UV) and even X-rays. The science behind photonics includes the following:
(i) emission, (ii) transmission, (iii) amplification, (iv) deflection, (v) detection using optical instruments and components, (vi) lasers and other light sources, (vii) electro-optical instrumentation, and even (viii) fiber optics.
Polymers are basically organic materials in the solid form, and essentially consist of a large number of monomers that are linked to each other in a repetitive manner. In electrical engineering, the thin polymer transistors have over the years gradually improved. This has resulted in low cost thereby enabling in the development and emergence of more flexible electronics. In RF-ID [radio frequency identification] tags, the recently engineered developments are in the areas of ink-jet printing, active-matrix display, and integrated circuits. Typical material used as a dielectric organic gate consists of spin-coated polyethersulfone (PES) that is doped, or reinforced, with Zn0.5Ni0.5Fe2O4 nanoparticles. Since solid polymer electrolytes, i.e., salt dissolved in a polymer matrix, suffer from a slow polarization response, the cross-linked ultra-thin polymer films used to develop polymer ion gels have shown incredible capacitance that often exceeds that of the conventional polymer insulators and even the ceramics chosen for use in organic thin-film transistors. Some polymer films, such as the bi-axially oriented polypropylene, provide high electrical insulation. This enables them to be used as a dielectric both in capacitors and other electronic components. Also, they can be used both as a sealant and as a encapsulate. Solar cells of the next generation are currently also being fabricated and put forth using polymer thin films.
In this research study, polyethersulfone thin films are reinforced with different concentration of the zinc-nickel ferrite (Zn0.5Ni0.5Fe2O4) nanoparticles [referred to henceforth in this paper as the ZnNiFe NPS]. Optical properties of the as-prepared samples were carried out using different spectroscopic techniques at room temperature (25°C). Structural properties, texture, and morphological characteristics of the as-prepared polymer thin films were studied with the help of the following:
(i) Fourier transform infrared (FTIR) spectra, (ii) X-ray diffraction (XRD), (iii) atomic force microscope (AFM), and (iv) scanning electron microscope (SEM).1–10 Spectroscopic studies have grown both in strength and confidence to become an imperative tool for the development of novel nonlinear molecules. The ground state dipole moment and excited state dipole moment of a chosen sample were determined so as to enable in an understanding of solute-solvent interactions that tend to occur in different environments.11 Among both the existing methods and available methods, the solvatochromic method does offer a high linear correlation between the spectroscopic parameters and functions of the chosen solvent.12–16
Polyethersulfone is a nucleophilic aromatic substitution between dichloro diphenyl sulfone and sodium salt of 1,4-dihydroxybenzene. Polyethersulfone can essentially be classified to be strong polymer that is suitable for high-performance applications. Polyethersulfone is an amorphous polymer that is capable of withstanding a wide range of temperatures without experiencing a noticeable change in material properties. These polymers are dimensionally stable in the environments of laboratory air and water. Therefore, they can be processed as thin films that form the basis of this independent research study. They are essentially a transparent polymer that find selection and use in electro-optical applications, such as photovoltaic cells. Besides having excellent electrical properties, they are also chemically resistant.
Material
The chemical formula of the polyethersulfone (PES) is (C12H8O3S) n, and its molecular weight is 232.26 g/mol. Polyethersulfone was acquired from TCI Chemicals [India] and had the following properties:
(i) density of 1.24g/cm3, (ii) melting point of 180°C, and (iii) refractive index of 1.615.
The molecular structure of PES is shown in Figure 1. The Zn (NO3)2, Fe (NO3)3, and ODH (Oxidative Dehydrogenation) were used for the preparation of nanoparticles.
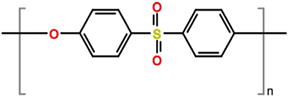
Figure 1 Molecular structure of polyethersulfone (PES)
Preparation of nanoparticles
The ZnNiFe nanoparticles were synthesized using the solution combustion method. The following steps were taken for preparation and/or synthesis of the ZnNiFe nanoparticles.
First, we measured the required amount of Zn (NO3)2, and Fe (NO3)3.
- Amount of Zn (NO3)2: 2.97grams
- Amount of Fe (NO3)3: 08grams
- Amount of ODH (Oxidative Dehydrogenation): 4.7257grams
The above chemicals were thoroughly mixed in a glass beaker. The resultant solution was mixed well using a magnetic stirrer at a speed of 550 rpm and temperature of 300°C. Along with this, 25ml of distilled water was added to the solution. We did observe a flame to arise as a direct consequence of smoldering. It took approximately 20 minutes to complete the reaction.
Doping with nickel (Ni+)
Here, we are adding Ni+ ions from the Ni nitrates of different concentration, i.e., x = 0.1, 0.3, 0.5, and 0.7, to get a different concentration of the nanoparticles. The different concentrations of the ZnFe oxides and oxidative dehydrogenation (ODH) are summarized in Table 1.
|
Ni-Nitrate (gm)
|
Fe Nitrate (gm)
|
Zn Nitrate (gm)
|
ODH (gm)
|
ZnNiFeO Nanoparticle
|
|
2.616
|
8.08
|
0.2974
|
4.722
|
Zn0.1Ni0.9Fe2O4
|
|
2.0346
|
8.08
|
0.8922
|
4.722
|
Zn0.3Ni0.7Fe2O4
|
|
1.4542
|
8.08
|
1.487
|
4.722
|
Zn0.5Ni0.5Fe2O4
|
|
0.8719
|
8.08
|
2.0818
|
4.722
|
Zn0.7Ni0.3Fe2O4
|
|
0.29
|
8.08
|
2.677
|
4.722
|
Zn0.9Ni0.1Fe2O4
|
|
0.29
|
8.08
|
2.677
|
4.722
|
Zn0.9Ni0.1Fe2O4
|
Table 1 Table of different concentration of ZnFe oxide and ODH
Production of a thin film
The ZnNiFe nanoparticles-reinforced polymer thin films were prepared using 0.5 percent of nanoparticles. To start with, the measuring beakers were thoroughly cleaned and subsequently dried in a hot air oven. Precision weighed three (3) portions of 0.10 mg of polyethersulfone (PES) granules using a weighing balance along with the following:
(i) 0.05 mg ZnNiFe (0.5%) nanoparticles, (ii) 0.10 mg ZnNiFe (0.5%) nanoparticles, and (iii) 0.15mg ZnNiFe (0.5%) nanoparticles.
The chosen portions were then mixed well in a beaker resulting in three polyethersulfone (PES) nanocomposites. An amount of 10 ml of dimethylformamide was added to the as-synthesized nanocomposites. The beaker was covered after placing a magnetic bead that was wrapped in an aluminum foil. The magnetic bead was placed on a magnetic stirrer. Temperature of the magnetic stirrer can vary between 45°C to -75°C, and speed of the magnetic stirrer was set at 700 - 900 rpm. The solution was allowed to stir for full 60 minutes. Herein, we are using the underlying concept of spin coating for preparing thin films of the chosen polymer. The spin coater [Model: EZ Spin A1 Spin Coater] housed within the research laboratory at the BMS Institute of Technology [Bangalore-560064, India] was used. The spin coater was calibrated for 10 minutes. The vacuum was switched on, the spinner was then thoroughly cleaned using acetone and then placed in the spin coater. Programing was done at a speed of 600 rpm for about 10 seconds, and the resultant outcomes saved. Three to four drops of the nanocomposite solution were allowed to gradually drip onto the glass substrate using a dipper. The spinner was then started and allowed to run very much in conformance with the program saved. This step is referred to as “Spin ON”. After 10 seconds, the spinner was turned off. This step is referred to as “Spin OFF”. The polymer thin film was then left to evaporate for about 15 minutes and subsequently placed in an air-tight container.
Solvatochromic shift method
In an attempt to understand the solute-solvent interactions and charge transfer characteristics of the solute in different environments, the electronic dipole moments of fluorophore in a liquid medium were evaluated using the equations put forth by Bilot and Kawaski.17,18 These equations are:
(1)
(2)
where
If the ground state
dipole moment and excited state
dipole moment is not collinear, then the angle between the two dipole moments
and
is determined using Equation (3)
(3)
Using the slopes obtained from the linear plots of Equation (1) and Equation (2), the dipole moment of the fluorophore in the ground state and excited state was determined using Equation (4) and Equation (5)
(4)
(5)
Using Equation (6) and Equation (7), the solvent polarity functions were determined.
(6)
And
(7)
As suggested by Lippert-Mataga,19 Bakhsheiv20 and Kawaski-Chamma-Viallet,21,22 we can determine dipole moment of the fluorophore in both the ground state and excited state using Equation (8), Equation (9) and Equation (10), as is given below.
(8)
(9)
(10)
The validity of Equation (8), Equation (9) and Equation (10) was checked using the solvent polarity function relations expressed by Equation (11), Equation (12), and Equation (13).
(11)
(12)
(13)
In these equations, n is the refractive index and ε represents dielectric constant of the solvent used. Further, m1, m2 and m3 are the slopes obtained by a linear fit of Equation (8), Equation (9) and Equation (10). The slope depends upon a change in the solute’s dipole moment upon excitation
and size of the cavity radius (a).
We can use Equation (14) and Equation (15) to determine
and
provided both are parallel to each other.
(14)
(15)
and
(16)
Suppaman’s expression (Equation 17) was used to determine radius of the cavity.
(17)
In Equation 17, N represents the Avogadro’s number, M represents the molecular wight and
represents density of the chosen solute.
Experimental techniques
A UV-Visible spectrophotometer [Model: Shimadzu UV Visible Spectrometer] was used to record Optical Density of the three doped thin films and pure polyethersulfone (PES). It was also used to record the absorption spectra of PES in the different solvent mediums. The Fourier transform infrared (FTIR) spectroscopy used in this research study was the Perkin Elmer FTIR and carried out at the research laboratory housed at the BMS Institute of Technology [Bangalore, India]. Use of the FTIR did reveal a noticeable change in the functional group of both the engineered thin films of the polymer composite and the pure polymer, i.e., polyethersulfone (PES). The photoluminescence study [using Model: FP-8300 Jarko] was carried out at the research laboratory housed within the campus of Mysore University [Mysore, India]. Fluorescence spectroscopy was done for the purpose of analysing fluorescent properties of the thin film samples.
The X-ray diffractometer [Model: D8 XRD] study was carried out at the Ramaiah Institute of Technology [Bangalore, India] and did reveal an observable change in intensity with concurrent improvement in crystallinity of the chosen engineered polymer nanocomposite.
The scanning electron microscope (SEM) [Model: Tescan Vegan 3] observations were carried out at the BMS College of Engineering [Bangalore, India] and did reveal an observable change in morphology of both the chosen polymer and the engineered polymer nanocomposites.
Atomic Force Microscopy (AFM) revealed overall roughness of the sample surface of the three engineered polyethersulfone thin films.
Fourier transform infrared spectroscopy (FTIR) results
The Fourier transform infrared spectra of the reinforced thin films were recorded over the frequency range (4000-400) cm−1. The thin film membranes that were prepared using the spin coating technique were found to be affected and/or influenced by the solvent used. Even if the solvent evaporated, both temperature and chemical properties of the solvent does exert an influence on the spin-coated membrane.23–28 In the FTIR spectra, 600 cm-1 to 1400 cm-1 is the fingerprint region, and the region from 1400 cm-1 to 4000 /cm-1 is the functional group region. The FTIR results for the chosen polyethersulfone polymer and the polyethersulfone / ZnNiFe nanocomposites for three different ratios of the ZnNiFe nanoparticles in 0.10 g of the chosen polymer [i.e., polyethersulfone (PES)] was (i) 0.5g ZnNiFe nanoparticles, (ii) 0.10g ZnNiFe nanoparticles, and (iii) 0.15g of ZnNiFe nanoparticles. Here we conducted FTIR in the mid-IR region, i.e., from 4500 cm-1 to 400 cm-1, for the four chosen thin films. The FTIR spectra of the four chosen thin films, namely:
(i) pure polyethersulfone (PES),
(ii) PES + ZnNiFe-1 [10g of polymer + 0.5g of nanoparticles),
(iii) PES + ZnNiFe-2 [10g of polymer + 0.10g of nanoparticles), and
(iv) PES + ZnNiFe-3 [0.10g of polymer + 0.15g of nanoparticles)
is shown in Figure 2. The –OH stretching vibrations appeared at 3527 cm-1 and 3366 cm-1 for the nanoparticle-reinforced thin films, and at 3174 cm-1 for pure polyethersulfone (PES). The C-H stretching and bending vibrations were observed at 2663 cm-1, 2584 cm-1, 2482 cm-1, 2417 cm-1, and 1588 cm-1, 1457 cm-1, and 1444 cm-1. We observed the -OH stretching band that arises due to the addition of ZnNiFe nanoparticles to disappear with a gradual increase in the percentage of nanoparticles in the chosen polymer PES (polyethersulfone). The regions of different wavelength peaks for the as-synthesized PES + ZnNiFe nanocomposites for different ratios of the nanoparticle reinforcements are summarized in Table 2.29
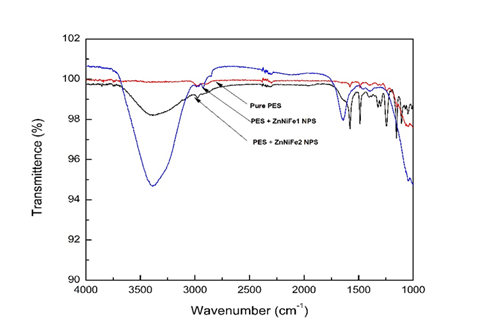
Figure 2 Fourier transform infrared (FTIR) spectra for pure polyethersulfone (PES), PES + ZnNiFe1 and PES + ZnNiFe2.
|
Wavenumber (cm-1)
|
Functional groups
|
|
3,527,313,133,663,170
|
-OH stretch, alcohols
|
|
2663, 2584,2482,2417
|
C-H stretch, alkane
|
|
2176, 2090
|
−C ≡ C − stretch, alkynes
|
|
194,718,441,837
|
C=O stretch, carbonyl group
|
|
158,814,571,444
|
CH bend, alkanes
|
|
1296
|
CN stretch, aliphatic amines
|
|
901,900
|
OH bend, carboxylic acid
|
|
741
|
OCN bending
|
|
553
|
Fingerprint region
|
Table 2 Fourier transform infrared (FTIR) table for the polyethersulfone nanocomposites
Ultra-violet [UV] visible spectroscopy
UV-Visible spectroscopy was conducted with the prime purpose of finding the Optical Density peak for the (i) pure polymers, (ii) pure ZnNiFe nanoparticles, and (iii) polymer/ZnNiFe nanocomposites. Basically, UV-visible spectroscopy was to determine the light absorption capability of the material. The absorption spectra of (i) pure PES, and (ii) PES + ZnNiFe nanoparticles, for different ratios of the ZnNiFe nanoparticles, was found to be constant for the three thin films that contained 0.5g of ZnNiFe nanoparticles, 0.10g of ZnNiFe nanoparticles, and 0.15g of ZnNiFe nanoparticles. We observed both Optical Density and the wavelength maxima at 339 nm to be unaffected by reinforcing polyethersulfone with ZnNiFe nanoparticles (Figure 3).30–33
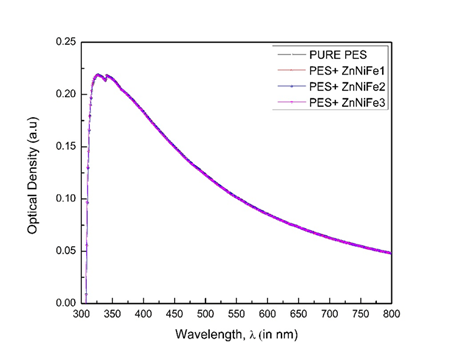
Figure 3 Typical absorption spectra of pure PES and the PES + ZnNiFe nanocomposites for different concentrations.
Photoluminescence results
Fluorescence (FL) emission spectroscopy was used to determine nature of the sample and to concurrently study and document the imperfection-related intensity of the fluorescence emission and peak wavelength depending on the conjoint and mutually interactive influences of nature of synthesis, solvent used, starting materials used, processing time, and few other factors.34–43 The graphs of photoluminescence for pure PES, pure Zn0.5Ni0.5Fe2O4 nanoparticle, and the engineered PES nanocomposite are shown in Figure 4. The optimum wavelength range is selected from 300 nanometers to 500 nanometers. For pure PES the peak intensity was observed to occur at 394 nm, and for the pure Zn0.5Ni0.5Fe2O4, nanoparticles it was observed to occur at 438 nm. For reinforcing the chosen polymer, i.e., PES, with nanoparticles, we do observe a noticeable increase in intensity of the as-synthesized PES nanocomposite and it is 409 nm. The photoluminescence intensity is directly proportional to the concentration of molecules. Hence, we can conclude that reinforcing the chosen polymer (i.e., polyethersulfone) was done properly. The observed increase in intensity for the as-synthesized polymer nanocomposites makes it a potential candidate for selection and use in Opto-electronic applications.
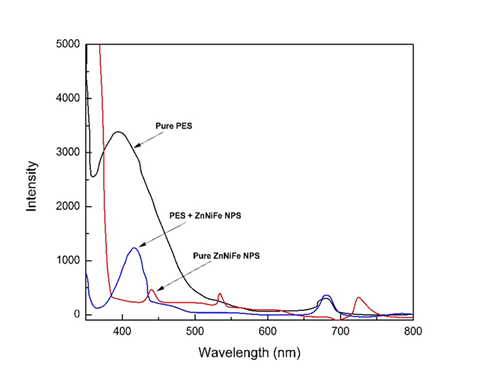
Figure 4 Photoluminescence graph for (i) pure PES, (ii) pure ZnNiFe nanoparticle and (iii) PES + ZnNiFe nanoparticle.
X-ray diffraction results
The x-ray diffraction [XRD] spectra of (i) pure polyethersulfone (PES), (ii) pure Zn0.5Ni0.5Fe2O4 nanoparticles, and (iii) reinforced polyethersulfone composites are shown in Figure 5. The XRD graph of polyethersulfone (PES) both with and without ZnNiFe nanoparticles is well displayed in Figure 5. It is observed that there are no distinct and observable peaks for the XRD spectra of pure polyethersulfone (PES). Hence, it can safely be concluded to be amorphous in nature. However, for the pure ZnNiFe nanoparticles a few sharp peaks were distinctly observed in the spectra. This goes to confirm an overall crystalline nature of the as-synthesized ZnNiFe nanoparticles. When polyethersulfone (PES) is combined with ZnNiFe nanoparticles a few high peaks did appear when compared one-on-one with the XRD spectra of the pure nanoparticles. This suggests that crystallinity has increased by the addition of ZnNiFe nanoparticles to polyethersulfone (PES).
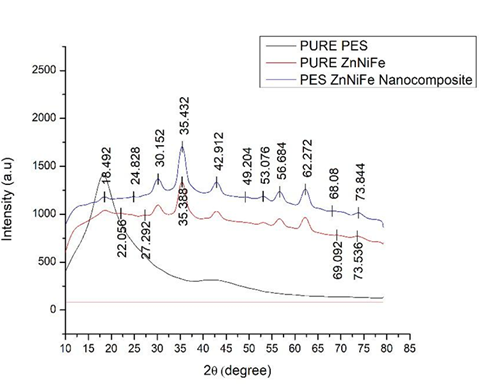
Figure 5 X-ray diffraction (XRD) graph of polyethersulfone (PES) both with and without ZnNiFe nanoparticles.
Atomic force microscopy [AFM] observations
The AFM images were taken to study roughness of the thin film surfaces of both the polymer (polyethersulfone) and polymer nanocomposite. Since the thin films were prepared using the spin coating technique, the thickness is less than that of the dip casting film. Consequently, roughness is less when compared one-on-one with the dip coating thin film having nanometer thickness. The 3-D images of pure polyethersulfone and the polyethersulfone nanocomposite are shown in Figure 6 and 7. It was observed that by reinforcing the chosen polymer, i.e., polyethersulfone, with ZnNiFe nanoparticles, the roughness of the thin film was noticeably reduced. By observing the images, we conclude that thickness of the thin film decreases with the addition of nanoparticles. The thickness was consistency for the thin films of pure PES and 1.3 μm and for the thin films of the PES nanocomposite it was 0.83μm. The surface texture, or morphology, of the thin films prepared for this research study is shown in Figure 8 and 9. Morphology of the pure polymer thin film was found to be of grainy texture, whereas morphology of the as-synthesized polymer nanocomposite was noticeably less granular when compared to pure polyethersulfone (PES). Also, we did observe an overall change in grain structure. The average grain size for pure PES was 1.0μm, whereas for the PES nanocomposite the average grain size was 0.8μm. This suggests that the approach chosen and used for the addition of nanoparticles to polymer polyethersulfone (PES) is correct and desirable.
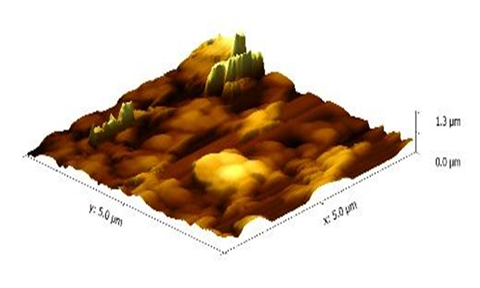
Figure 6 Atomic force microscope (AFM) 3-D (three-Dimensional) image of pure polyethersulfone (PES).
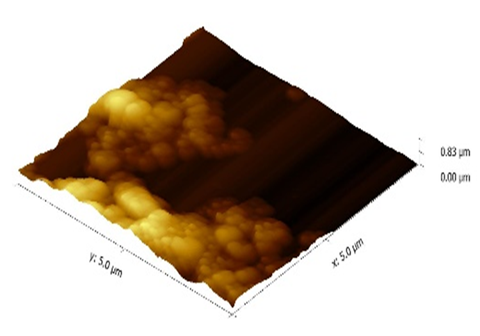
Figure 7 Atomic force microscope three-dimensional image of the polyethersulfone (PES) nanocomposite.
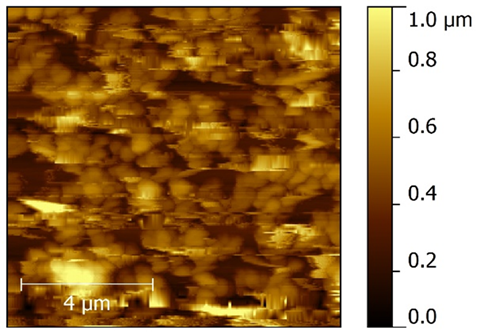
Figure 8 Atomic Force Microscope morphology of pure polyethersulfone (PES).
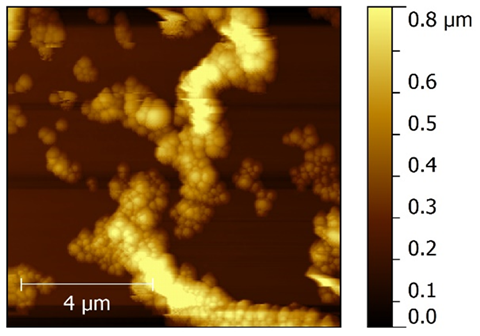
Figure 9 Atomic force microscope (AFM) morphology of the polyethersulfone (PES) nanocomposite.
Scanning electron microscopy observations
The Scanning Electron Microscope (SEM) images of pure polyethersulfone (PES), pure ZnNiFe nanoparticles, and PES + ZnNiFe nanocomposite are shown in Figure 10–12. Size of PES is 2μm and size of the ZnNiFe nanoparticle is 500 nm. The difference in grain size can be distinctly seen. A noticeable change in morphology occurs when ZnNiFe nanoparticles are added to the polymer (PES) and the size is around 1μm as shown in Figure 12. For pure polyethersulfone (PES) (Figure 10), it is granular in structure with a lot of spacing, or less networking, within the structure. This reveals a lot of space between the particles. However, for the pure nanoparticle, it is a highly networked structure with not many gaps or “open” spaces. The addition of ZnNiFe nanoparticles to the chosen polymer (polyethersulfone), we did observe a network-like structure with no gaps. Further, the network was both pronounced and noticeable.

Figure 10 Scanning electron microscope image of pure polyethersulfone.

Figure 11 Scanning electron microscope image of pure Zn0.5Ni0.5Fe2O4.

Figure 12 Scanning electron micrograph of the polyethersulfone + Zn0.5Ni0.5Fe2O4 nanocomposite.
Estimation of the ground state dipole moment and excited state dipole moment of polyethersulfone (PES)
The PES molecule's typical absorption and fluorescence spectra were recorded in different solvents and are shown in Figure 13 and 14. Values of the dielectric constantand
polarity parameters of the different solvents are summarized in Table 3. The solvatochromic data of polyethersulfone (PES) in the different solvents are summarized in Table 4. It is observed that the fluorescence wavelength maxima undergo a bathochromic effect on increasing solvent polarity. This can essentially be attributed to a
transition at the spectral level. A greater magnitude of Stokes shift reveals geometry of the excited state to be different from that of the ground state coupled with the nature of intermolecular charge transfer (ICT) of the excited state. The plots of Bilot-Kawaski’s, Lippert’s, Bakshiev’s, and Kawski-Chamma-Viallet’s were plotted using the Origin 8 software and is as shown in Figure 15–18. A statistical treatment of the correlations for solvent spectral shifts of the PES molecule is provided in Table 5. For the Bilot-Kawaski method, values for
and
were obtained and 3.623 D and 6.859 D for a molecule of the chosen polymer (polyethersulfone). The dipole moment in the ground state and dipole moment in the excited state
was determined using Equation (17) and Equation (19) and found to be 1.916 D and 5.153 D for a molecule of PES. The dipole moment for the excited state obtained from use of the Lippert’s equation, Bakshiev’s equation, and Kawski-Chamma-Viallet’s equation are 7.401 D, 5.153 D, and 6.699 D for a molecule of PES. The angle between
and
, i.e.,
, was found to be 8 degrees for a molecule of PES. These values are neatly summarized in Table 6. From this table (Table 6) it is observed that values of the excited-state dipole moment are noticeably greater than that of the ground state dipole moment for a molecule of the polymer polyethersulfone (PES). From these observations, we conclude that a molecule of the chosen polymer (PES) is more polar in the excited state than in the ground state. However, the values of
are marginally different for the different models chosen and studied. This is essentially because the different models use different assumptions for the development of their model. It is observed that an increase in dipole moment in the excited state can be attributed to specific solvent effects, such as the hydrogen bonding effect.
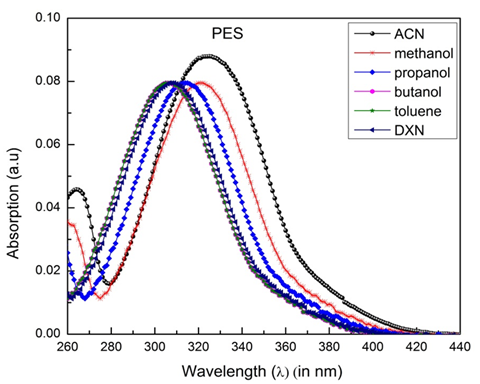
Figure 13 The absorption spectra of the polyethersulfone (PES) molecule in different solvents at room temperature.
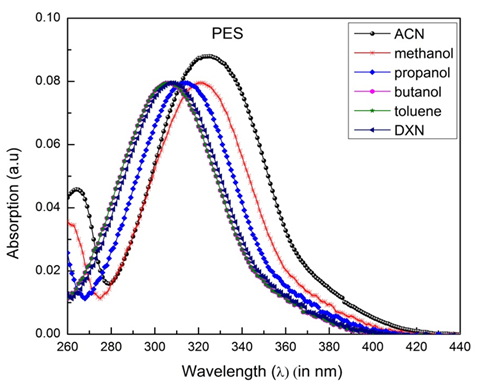
Figure 14 The fluorescence spectra of a molecule of polyethersulfone (PES) in the different solvents at room temperature.
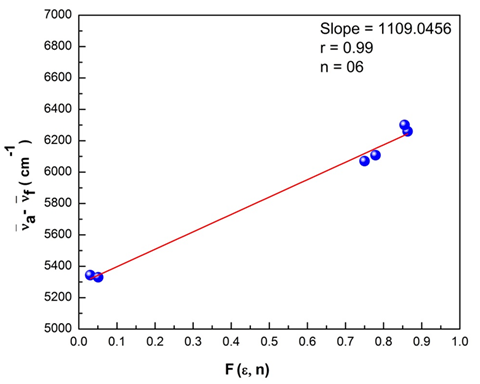
Figure 15 The stoke’s shift as a function of F (e, n) for the polyethersulfone (PES) molecule.
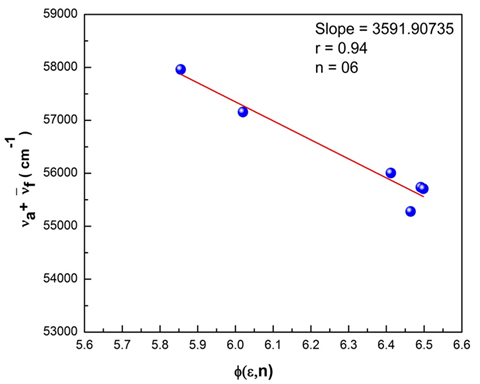
Figure 16 The Stoke’s shift as a function of f(e, n) for the polyethersulfone (PES) molecule.
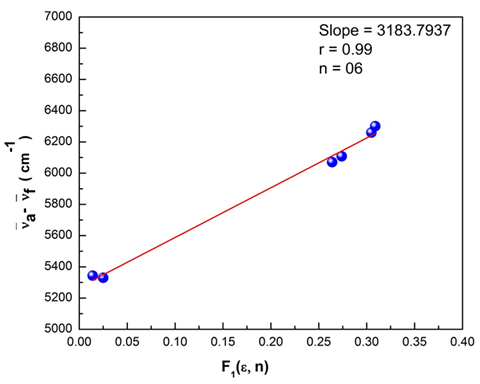
Figure 17 Lippert’s plot for the polyethersulfone (PES) molecule.
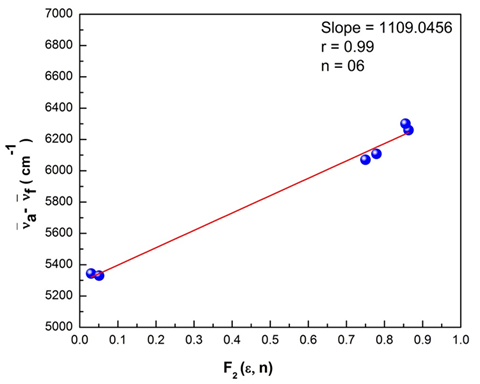
Figure 18 Bakshiev’s plot for the polyethersulfone (PES) molecule.
|
Sl. No.
|
Solvents
|
|
|
|
|
|
|
|
1
|
ACN
|
37.5
|
0.305
|
0.863
|
0.666
|
2.8
|
6.464
|
|
2
|
methanol
|
32.7
|
0.309
|
0.855
|
0.651
|
2.778
|
6.412
|
|
3
|
propanol
|
20.33
|
0.274
|
0.779
|
0.652
|
2.856
|
6.491
|
|
4
|
butanol
|
17.5
|
0.264
|
0.75
|
0.646
|
2.874
|
6.498
|
|
5
|
toluene
|
2.38
|
0.014
|
0.03
|
0.35
|
2.995
|
6.02
|
|
6
|
DXN
|
2.25
|
0.025
|
0.051
|
0.311
|
2.902
|
5.855
|
Table 3 The values of dielectric constant and polarity parameters of different solvents
|
Sl. no.
|
Solvents
|
|
|
|
|
|
|
|
|
1
|
ACN
|
325
|
408
|
30769.2308
|
24509.8039
|
6259.42685
|
55279.0347
|
27639.5174
|
|
2
|
methanol
|
321
|
402
|
31152.648
|
24852.186
|
6300.4616
|
56004.834
|
28002.417
|
|
3
|
propanol
|
323
|
403
|
30921.6658
|
24813.8958
|
6107.77
|
55735.5616
|
27867.7808
|
|
4
|
butanol
|
316
|
391
|
31645.5696
|
25575.4476
|
6070.12205
|
57221.0172
|
28610.5086
|
|
5
|
toluene
|
320
|
386
|
31250
|
25906.7357
|
5343.2643
|
57156.7357
|
28578.3679
|
|
6
|
DXN
|
316
|
380
|
31645.5696
|
26315.7895
|
5329.78013
|
57961.3591
|
28980.6795
|
Table 4 Solvatochromic data for a molecule of polyethersulfone (PES) in the different solvents
|
Correlations
|
Slope (m)
|
Correlation factor (r)
|
Number of solvents (n)
|
|
Bilot Kawaski’s correlation
|
3591.907
|
0.94
|
6
|
|
Lippert’s
|
3183.793
|
0.99
|
6
|
|
Bakshiev’s
|
1109.045
|
0.99
|
6
|
|
Kawski-Chamma-Viallet’s
|
2421.835
|
0.87
|
6
|
Table 5 Statistical treatment of the correlations of solvents spectral shifts for a molecule of polyethersulfone (PES)
|
Compound
|
|
|
|
|
|
|
|
|
|
|
|
PES
|
4.546
|
3.623
|
1.916
|
6.859
|
5.153
|
7.401
|
5.153
|
6.699
|
5.485
|
80
|
Table 6 Ground state dipole moment and excited state dipole moment of the polyethersulfone (PES) molecule
athe ground state dipole moment calculated from Bilot-Kawaski Eqn. (4),
bthe ground state dipole moment calculated using Eqn. 17,
cthe excited state dipole moment calculated using Bilot-Kawaski Eqn. (5),
dthe excited state dipole moment calculated using Eqn.19,
ethe experimental excited state dipole moment calculated from Lippert’s equation,
fthe experimental excited state dipole moment calculated from Bakshiev equation,
gthe experimental excited state dipole moments calculated from Kawaski-Chamma-Viallet equation,
hthe change in dipole moments for μe and μg,
iThe angle between ground state dipole moment and excited state dipole moment.










