eISSN: 2574-9927


Review Article Volume 7 Issue 4
1Department of Chemistry & Environmental Science, New Jersey Institute of Technology, USA
2Interdisciplinary Program in Materials Science & Engineering, New Jersey Institute of Technology, USA
Correspondence: Nuggehalli M Ravindra, InterdisciplinaryProgram in Materials Science & Engineering, New Jersey Institute of Technology, Newark, NJ 07102, USA, Tel 9735963278
Received: October 28, 2023 | Published: November 13, 2023
Citation: Ravindra NM, Brenckman C, Hossain S. Nanotechnology-based drug delivery systems for treatment of knee injuries and Alzheimer’s disease – a review. Material Sci & Eng. 2023;7(4):166-179. DOI: 10.15406/mseij.2023.07.00220
The effective treatment of human illnesses relies on the ability to deliver therapeutic compounds to diseased sites in a highly efficient manner. However, conventional therapeutic strategies often require high systemic administration due to non-specific biodistribution and rapid metabolism of free drug molecules before they can reach their intended targets which leads to undesirable side effects. Nanotechnology offers a solution to mitigate these issues by allowing the development of drug delivery systems (DDS) within the nanometer size range, which can alter the pharmacological and therapeutic effects of drug molecules. These novel DDS have several advantages over traditional large-scale systems, including altered pharmacokinetic behavior and improved payload, owing to their small size. Moreover, their surface chemistry can be easily modified to attach targeting and therapeutic molecules for specific curative and therapeutic applications. In this comprehensive review, we explore and present the potential of nanoparticles as a highly effective drug delivery system for the treatment of knee injuries and Alzheimer’s disease. With the aim of addressing the challenges that are associated with drug delivery, nanotechnology has emerged as an increasingly important area of research. Scientists have conducted extensive investigations into nanosystems with varying compositions and biological properties for use in drug delivery applications. In order to ensure efficient drug delivery, it is critical to understand how nanomaterials interact with the biological environment, target cell-surface receptors, release drugs, administer multiple drugs, and maintain the stability of therapeutic agents. The recognition and appreciation of the molecular mechanisms that are involved in cell signaling in relation to the disease under investigation is of fundamental importance.
Keywords: nanotechnology, biological, alzheimer’s disease, nanosystems
ADAMTS-5, A disintegrin and metalloproteinase with thrombospondin motifs 5 2; CrmA, cytokine response modifier A; DPPC, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; HA, hyaluronic acid; HDI, hexamethylene diisocyanate; IL, interleukin; MMP, matrix metalloproteinase; NGF, nerve growth factor; OARSI, osteoarthritis research society International; PAE, poly(β-amino ester); PEAs, poly(ester amide)s; PEG, poly(ethylene glycol); PLA, poly(lactic acid): PLGA, poly(lactic-co-glycolic acid); pNIPAM, poly(N-isopropylacrylamide); TNF-α, tumor necrosis factor alpha; WBC, white blood cells
Nanotechnology is an area of science and engineering that deals with the design, production, and manipulation of materials at the nanoscale level (typically between 1 and 100 nanometers). The application of nanotechnology in medicine is known as Nanomedicine, which involves the use of nanoscale materials and devices for the diagnosis, treatment, and prevention of diseases. Nanotechnology has enabled the development of novel materials and devices with unique physical, chemical, and biological properties that are useful in various medical applications. In nanomedicine, nanoparticles, nanotubes, and nanorods have been designed to target specific cells or tissues, cross biological barriers, and deliver drugs or therapeutic agents with high precision and efficiency. One of the most significant benefits of nanomedicine is the ability to deliver drugs and therapeutic agents directly to the site of disease. This targeted drug delivery approach can improve the effectiveness of treatments while minimizing side effects.1
Nanotechnology is also being used to develop diagnostic tools that can detect diseases at an early stage, allowing for earlier intervention and better outcomes. In addition to drug delivery and diagnostics, nanotechnology is being explored for other medical applications, such as tissue engineering, wound healing, and regenerative medicine. For example, nanoscale scaffolds can be used to promote the growth of new tissue in damaged or diseased areas of the body.2,3 Overall, nanotechnology holds great promise for revolutionizing the field of medicine. The continued development of nanoscale materials and devices is likely to lead to new and improved treatments for a wide range of diseases and conditions.
Drug delivery system can be defined as a design or a device that expedites the introduction of a therapeutic substance into the body with the intent to progress its efficacy and safety. This can be achieved by regulating the rate, time, and place of release of the drug within the body. In order to improve efficacy, the interaction of the drug with its target (cell receptors) and rate, time and place of release must be improved.4 Nanotechnology has revolutionized drug delivery systems by allowing drugs to be targeted directly to specific cells or tissues in the body. The use of nanotechnology in drug delivery systems has several advantages, including improved drug solubility and stability, protection of drugs from degradation and clearance by the body, controlled drug release, allowing for sustained or targeted drug delivery, reduced toxicity and side effects, and enhanced therapeutic efficacy.4
The most common types of materials used in nanotechnology are nanoparticles (NP), which contain substances in the form of particles. NPs are composed of three structural parts which include the surface layer, the shell layer and the core. The surface layer may be functionalized with a variety of small molecules, metal ions, surfactants, and polymers. The shell layer is a structure with chemically diverse material. Lastly, the core is essentially the central part of the NP.5 NPs are characterized based on their chemical properties as either organic, inorganic, or carbon-based. Organic NPs utilize noncovalent interaction for the self-assembly and design of molecules to transform into specific desired structures, which include dendrimers, micelles, liposomes and ferritin or polymers. Organic NPs are very specific due to their biodegradable and non-toxic effect. Inorganic NPs are not made up of a carbon skeleton and chiefly use metal ions and metal oxides for formation. They carry very distinctive properties such as size, high surface area to volume ratio, pore size, surface charge/density, crystalline/amorphous structures, and spherical/cylindrical shapes. They are sensitive to atmospheric factors such as air, moisture, heat, and sunlight. Lastly, carbon-based NPs are principally made up of carbon particles that show several individual chemical, physical, mechanical and thermal properties.5 A schematic of the various applications of nanoparticles in medicine is shown in Figure 1. This comprehensive review will focus on nanotechnologybased drug delivery systems for successful outcomes for: (a) knee joint injuries and (b) Alzheimer’s disease management.
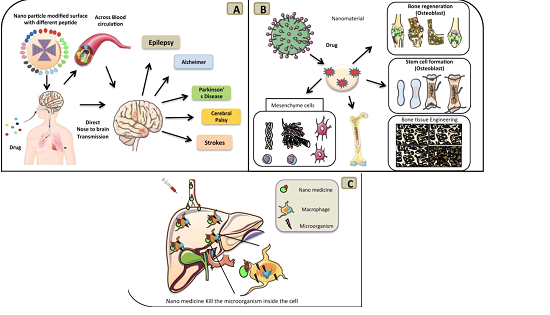
Figure 1 (A) Displays a modified nanoparticle that contains a different surface peptide that is inhaled through the nose and goes to direct transmission in the brain, (B) Shows a nanoparticle being directly used as a drug carrier to stimulate mesenchyme cell induction, bone cell regeneration, stem cell formation and bone tissue engineering, (C) Displays a nanoparticle being injected into the blood and the process of it going directly to liver/hepatic cell and kills the microorganism.
The most popular routes of administration for drug delivery systems include injections, oral, inhalation and transdermal methods. However, there are other different routes of administration such as injections, ocular and nasal. For a small molecule drug, the most common form of delivery is via the oral route. Worldwide, tens of billions of pills are consumed for just aspirin alone. Oral pills are convenient and most compliant for patients due to their pre-determined and measured doses, movability, distinct dosing times and inclusive non-invasive type of administration. However, they do not have the capability to deliver larger therapeutic molecules such as proteins and peptides. Other disadvantages to oral administration include reduced bioavailability, nontargeted nature, and can be potentially unpredictable due to the presence of food.6
For protein and peptide administration, the most common form of delivery is by injections. On average, more than ten billion injections are performed each year worldwide. Injections are beneficial because they are rapid and onset, have up to 100% bioavailability, have a controlled depot release and are suitable for most therapeutic molecules. However, injections are difficult for patients to administer to themselves; a massive number of patients fear needles leading to noncompliance, and there is a greater risk of infection.6 The most used routes of administration for drug delivery, emphasizing their advantages, disadvantages, and potential targets are summarized in Table 1. Research on DDS is focused on improving oral and injectable systems and on opening supplementary routes of administration such as pulmonary, transdermal, ocular, and nasal routes.6
|
Route of Administration |
Advantages |
Disadvantages |
Systems |
|||
|
Oral |
· |
Non-invasive |
· |
Drug absorption may vary Subject to first-pass metabolism |
· |
Pills |
|
· |
Cheap |
· |
Unsuitable for unconscious or vomiting patients |
· |
Liquid |
|
|
· |
Safe |
· |
Slow onset action |
Medications |
||
|
· |
Simple and convenient |
· |
The drug may be destroyed by digestive enzymes and/or stomach acid |
|||
|
· |
Self-administration |
· |
||||
|
Injections (IV and IM) |
· |
Immediate Effect |
· |
Risk of infection |
· |
Vaccines |
|
· |
Avoids first-pass metabolism |
· |
Expensive |
· |
Insulin |
|
|
· |
Up to 100% bioavailability |
· |
Difficult to self-administer |
· |
Chemotherapy |
|
|
· |
Incompliance |
|||||
|
Pulmonary/Inhalatio n |
· |
Rapid onset action Systematic side effects minimized |
· |
Proper inhaler technique rewired for the drug to work maximally Only a small number of drugs can be given this route |
· |
Inhalers |
|
· |
Reached the site of action |
· |
May stimulate cough reflex |
· |
Anesthetics |
|
|
· |
· |
|||||
|
Transdermal |
· |
Convenient |
· |
Expensive |
· |
Patches |
|
· |
Long duration of drug action Usually requires less frequent application |
· |
Local irritation |
· |
Creams |
|
|
· |
Can be self-administered |
· |
Gels |
|||
|
· |
Avoids first-pass metabolism |
|||||
|
· |
Steady plasma concentration |
|||||
|
· |
Slow absorption |
|||||
|
· |
||||||
|
Ocular |
· |
Convenient for patient Systematic side effects reduced |
· |
May cause temporary blurring of vision |
· |
Eye Ointment Eye Drops |
|
|
· |
|
· |
Barriers to administration (Ex. Poor manual dexterity, poor vision) |
· |
|
Table 1 The most used routes of administration for drug delivery, including their advantages, disadvantages, and systems
Nanotechnology-based drug delivery systems for knee joint injuries
The knee joint is a highly intricate synovial joint that boasts remarkable mobility. It consists of various components, including the synovial membrane, articular cartilage, ligaments, menisci, tendons, and patella.7 These elements work together seamlessly to provide biomechanical support. To ensure that the knee remains stable, it relies on six ligaments, numerous tendons, muscles, and cartilaginous structures. One such example is the cruciate ligament, which prevents the tibial bone from shifting forward or backward.8 The knee joint contains two menisci, which are c-shaped pieces of cartilage that act as cushions and absorb shock between the femur (thigh bone) and tibia (shin bone). These menisci provide mechanical stability within the knee joint capsule during movement.9 Despite the inherent strength of the knee joint, various factors can contribute to injuries in people of all ages. These include sports, repetitive stress, accidents, rheumatoid arthritis (RA), osteoarthritis (OA), trauma, and aging.10 There are many types of knee joint injuries such as contusions, sprains and strains, as well as injuries to the ligament, cartilage, tendon, and meniscus tear, in addition to synovial inflammation.11–13 The most common degenerative disease to affect the knee is osteoarthritis.14 Studies revealed that between 1990 and 2019, there was a significant rise of 113.25 percent in the number of people affected by osteoarthritis worldwide, increasing from 247.51 million to 527.81 million. The age-standardized prevalence rates (ASRs) in 1990 and 2019 were 6,173.38 and 6,348.25 per 100,000, respectively, indicating an average annual increase of 0.12 percent.15
Several nanomedicines have been recognized for treating knee joint injuries. Figure 2 represents the main types of knee-joint related injuries. Nanomedicines provide therapeutics with tremendous penetration and retention rates in a controlled manner when delivered at the site of action. They typically possess suitable properties such as high biodegradability, non-toxicity, biocompatibility, high drug encapsulation with the capability of precise site-specific delivery, etc., all of which have the potential to advance treatments that are already established. Nanomedicines can be created from a variation of materials including synthetic polymers, biopolymers and naturally occurring materials such as polysaccharides and proteins. However, natural materials are more beneficial due to their homology to the structure of cartilage, tendon and other aspects that are associated with the knee joint. Most used as the nanocarrier to construct the nanomedicines and their structures include nanoparticles, dendrimers, liposomes, micelles, exosomes etc. These nanocarriers support therapeutics in relieving symptoms such as pain and inflammation and progress the function of the knee joint by repairing it or healing it.16
Administration
The site of administration has a direct effect on the mechanism of action and outcome and therefore is a critical aspect to consider. Typically, Intra-articular (IA) injection is a method utilized to achieve site-specific outcomes. It is beneficial because it has the ability of increasing local drug concentration at the site of action, which reduces drug dosage and mitigates traditional side effects caused by the drugs. Additionally, it can assist the delivery of drugs with low oral bioavailability, such as inhibitory RNAs, recombinant proteins and therapeutic genes. Due to the lack of vascular structure in cartilage and meniscus, and the adverse effects to other healthy organs, oral, transdermal, and other systematical administration techniques may not be as effective as IA injection for cartilage and meniscus repair.16
Major types of nanotechnology-based drug delivery systems for knee joint injuries
Nanoparticles
The structures of nanocarrier systems are shown in Figure 3. Nanoparticles are a common form of dosage for the delivery of drugs to improve release profiles, targeting, combined diagnosis, treatment and overcoming multidrug resistance.17 The NPs can be found in the shapes of spheres, needles, cubic bars, cages, and prisms that can carry small molecular drugs, proteins, stem cells, etc. to the targeted site.18 NPs are beneficial when compared to conventional drugs because they improve the delivery of insoluble drugs, increase bioavailability, reduce side effects, and enhance treatment effectiveness.17 Novel cartilage affinity peptide (CAP, DWRVIIPPRPSA) -modified nanoparticles (Gd2(CO3)3@PDA-PEGDWpeptide) coated with polydopamine (PDA) were created by Ouyang and group that presented significant decrease in apoptosis and inflammation and increased maturation of chrondrocytes in vitro.19 In vivo assessments suggest that the NPs are a robust and efficient bioplatform for the delivery of remedial agents for the treatment of general cartilage-related diseases such as osteoporosis as the biodistribution is concentrated in cartilage and liver compared to other tissues and all the test animals experience a steady increase in body weight through the entire duration of the experimental procedure indicating minimal cytotoxicity. Red blood cells count from test animals and control showed no difference, and accessory organs such as the liver, gall bladder and kidneys exhibited normal functional activities.19 Silver nanoparticles (AgNPs) derived from the reduction of silver ions in the extract of Bauhinia acuminate flowers have been shown to promote healing in meniscus injuries by improving the differentiation of mesenchymal stem cells (MSCs) into osteoblasts. In vivo results showed reduction in inflammation and increased growth of new tissue; thus, it could be a promising approach for the treatment of osteogenesis as well.20 Tendon mechanical strength, ECM synthesis, and tissue repair and regeneration have also been shown to improve by utilizing single-walled carbon nano-horns (CNH), silver nanoparticles, and nonviral vectors for gene delivery, respectively.21–23

Figure 3 The structures of nanocarrier systems including: (a) nanoparticles, (b) dendrimer, (c) liposome, (d) micelles and (e) exosome.16
Dendrimers
Another form of dosage for drug delivery includes dendrimers, which are tree-like molecules that contain a well-branched and defined structure. They are typically used as nanocarriers for the delivery of a wide variety of drugs and bioactive compounds. Dendrimers can be utilized to treat numerous knee injuries because they aid in protecting the injury site, reduce inflammatory cytokines and minimize the side effects of other medicines. Additionally, they can improve targeted drug delivery, elevate PK parameters, and regulate drug release at the injection site.16–24 Kartogenin (KGN), a small molecule capable of enhancing chondrogenic differentiation of human mesenchymal stem cells (hMSCs), has shown promise in cartilage regeneration at the tendon-bone junction when injected directly into intact rat patellar tendons in vivo with negligible potential for systemic toxicity, evidenced by low concentrations of the molecule in the intra-articular space.25,26 Zhang and group demonstrated that direct injection at wound site might not be the optimal mode of delivery as it is difficult to limit the KGN to its intended location without a scaffold.25 Thus, a platelet-rich plasma bioscaffold, used in tandem with the KGN, can help prevent its spread to neighboring areas where it can form excessive cartilage-like tissues in unintended areas.25 Geiger and group have identified a charged PEGylated amine terminal polyamidoamine (PAMAM) dendrimer conjugated to growth factor 1 (IGF-1) that showed 70% uptake into cartilage tissue and 100% cell viability.27 Coating with PEG allows for control of the charge density on the surface of the molecule. It was shown that the dendrimer penetrated ex vivo bovine cartilage within 2 days and improved the delivery of the growth factor with therapeutic benefit to cartilage, bone, and synovium in a rat model of osteoarthritis.27 Histotoxicity studies at 2 and 7 days showed no greater toxicity than the control and all toxicological biomarkers were reported within normal range or statistically equivalent to uninjected animals. At two months, histological analysis for chronic organ damage showed normal functionality of joints, liver, kidney, and lungs.27 One limitation of this study was the large hydrodynamic radius of the PEGylated dendrimer which caused 30% of the total injected dose to be excreted by urine within the first 7 days.27 These findings imply that a partially PEGylated dendrimerdrug conjugate could enhance the effectiveness of both present and future osteoarthritis medications; however, conducting preclinical research on larger animals is essential for regulatory approval.
Liposomes
Liposomes can be used as another form of dosage for DDS. They contain a hydrophilic core that can encapsulate hydrophilic drugs and they contain an outer bilayer that can carry lipophilic agents/drugs.16 Liposomes can be either fabricated with functional materials or modified with targeting agents. The encapsulation of drugs in liposomes can reduce drug toxicity with augmented drug effectiveness.28 Despite being a highly promising drug delivery system, liposomes have some drawbacks such as drug preleakage, incomplete and rapid drug release, among others, which require further investigation to enhance their performance.16 Ai and group have engineered a collagen targeting nanodelivery platform using ultra-small lipid-polymer hybrid nanoparticles (LP-NPs) with a diameter of around 25 nm consisting of a core made from poly(lactic-co-glycolic acid) (PLGA) and a shell from polyethylene glycol (PEG)modified lipid.29 It was further conjugated with a short collagen-binding peptide (WYRGRLC) that allowed for nanoparticle entrapment in the Extracellular Matrix (ECM) and loaded with an activator called MK-8722 which functionalizes a protein kinase that regulates chondrocytes energy metabolism in cartilage.30,31 The LP-NPs designed to target collagen, were able to release drugs consistently for 48 hours without any sudden bursts. After injection into the knee joints of mice with collagenase-induced osteoarthritis, it was found that the ultrasmall size and collagen-targeting ability together allowed enhanced retention of LP-NPs and followed a diffusion-controlled drug release mechanism. Proinflammatory cytokine levels were down and an enlarged synovial lining layer with a higher cellular density was observed at day 13. This suggests alleviation of osteoarthritis symptoms Xue et al.,29 developed a liposome that targeted the delivery of three anti-rheumatic agents: dexamethasone, NF-κB decoy oligodeoxynucleotides, and gold nanorods.32 In conjunction with laser irradiation, this treatment effectively reduced the production of proinflammatory proteins and in vitro oxidative factors. In an adjuvant-induced mouse model, the formulation was found to accumulate in inflamed paws for a longer period of time and at a higher rate. This therapy was also able to decrease clinical arthritis scores, serum cytokine levels, and preserve cartilage. The study further observed a reduction in the expression of proinflammatory proteins (TNF-α and IL-6) and NO. These findings highlight the significant potential of liposomes as a means of treating inflammatory arthritis through a combination of anti-inflammatory mechanisms.32 Chen et al. developed a novel liposomal drug delivery platform that targets activated macrophages via folate receptor-mediated endocytosis and releases the drug via oxygen generation-induced structural failure of the liposome through catalyzed reaction between catalase and elevated intracellular H2O2 for the treatment of rheumatoid arthritis (RA).33 The liposomes were prepared using folate-PEG (100) monostearate (FOL-S100); the folate component is responsible for receptor targeting, while the presence of PEG helps extend the blood circulation time of the liposomes and enhances their penetration into inflamed joints through the enhanced permeability and retention (EPR) effect. The liposomes were composed of POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) and were designed to encapsulate enzymes such as alcohol dehydrogenase, catalase, and glucose oxidase. Methotrexate and catalase were co-encapsulated into the liposomes, with the former serving as an antirheumatic drug and the latter acting as a trigger for ROS-responsive drug release and as an antioxidant to balance the redox state. The formulations exhibited sustained drug release in saline, releasing greater than 86% of their contents. This is thought to be due to the continuous production of oxygen resulting from the reaction between the catalase and H2O2 inside saline solution that causes failure or collapse of the carrier structure. In vivo biodistribution studies revealed that there was reduced drug accumulation in the liver and spleen compared to the non-liposomal drug formulation, which indicates that the PEGylation of the liposomal surface was effective in reducing their uptake by the reticuloendothelial system (RES). The liposomal formulation accumulated extensively in inflamed joints, up to 6.79-fold higher for some formulations when compared to the free drug solution.33 These results make liposomes a promising strategy for improving drug delivery to inflamed joints in conditions such as arthritis.
Micelles
Another form of dosage for delivery is micelles, which are amphiphilic and self-assembling nanocarriers. They are capable of absorbing poorly water-soluble drugs and attaining continuous or managed drug release. Micelles protect drugs from degradation and prompt clearance. They can encapsulate or create within themselves many small hydrophobic molecules, herbal extracts, vectors, and genes. They are exceptionally favorable for synthetic and local drug targeting due to their small size, persistent drug release kinetics, and manageable degradation under physiological environments.34 She et al., prepared dextran sulfate-triamcinolone acetonide (DS-TA) conjugate NPs for targeted treatment of osteoarthritis.35 These DS-TA NPs were designed to target scavenger receptor class A (SR-A) that is present on activated macrophages. Inflammation associated with macrophages plays a significant role in the development and worsening of osteoarthritis (OA), leading to the manifestation of symptoms and acceleration of disease progression.36,37 Flow cytometry and confocal laser scanning microscopy analyses confirmed the precise targeting specificity of DS-TA NPs towards SR-A. The viability of activated macrophages (RAW 264.7 cells) was effectively reduced, along with the expression of proinflammatory cytokines, demonstrating the efficacy of DS-TA NPs. The intra-articular injection of DS-TA NPs was able to alleviate structural damage to joint cartilage, as indicated by histopathological analysis. Moreover, the expression of proinflammatory cytokines (including IL-1β, IL-6, and TNF-α) in the cartilage tissue was significantly reduced by DS-TA NPs. These findings suggest that DS-TA NPs have significant potential as a targeted therapeutic nanomedicine for the treatment of OA.35
Exosomes
The last form of drug dosage for delivery is exosomes, which are membrane-based nonimmunogenic vesicles acquired from certain cells. They are an ideal DDS because of their biocompatibility, high targeting, least toxicity and natural pharmacological effects. They are nanovesicles and contain bioactive lipids, proteins, and microRNAs in their structure. This gives them the ability to serve as messengers between cells for the passage of biological signals to recipient cells to repair injured or diseased cells. Exosomes have no tumorigenicity and immunogenicity which make them a better delivery system to treat knee injuries or knee-related diseases.16–38 Wang et al.,39 harvested exosomes from human embryonic stem cell-induced mesenchymal stem cells (ESC-MSCs) and created an in vitro model by treating primary mouse chondrocytes with interleukin 1 beta (IL-1β). The effects of the conditioned medium with or without exosomes and varying doses of isolated exosomes were evaluated through immunocytochemistry and western blot analysis after 48 hours. To create an OA model, destabilization of the medial meniscus (DMM) surgery was performed on the knee joints of C57BL/6 J mice. Intra-articular injection of either ESC-MSCs or their exosomes was then administered. Histological staining and OARSI scores were used to assess cartilage destruction and matrix degradation at 8 weeks’ post-surgery. The study showed that administering ESC-MSCs through intra-articular injection improved cartilage destruction and matrix degradation in the DMM model. It was also found that the beneficial effect was mediated by exosomes derived from the ESC-MSCs. In vitro experiments revealed that these exosomes enhanced collagen type II synthesis and reduced ADAMTS5 expression in the presence of IL-1β, thereby maintaining the chondrocyte phenotype. Immunocytochemistry demonstrated that the exosomes and collagen type II-positive chondrocytes were co-localized. Furthermore, administering exosomes derived from ESC-MSCs through intra-articular injection effectively prevented cartilage destruction in the DMM model.39 Wang et al., established a model of rat Achilles tendon tendinopathy by injecting CollagenaseI, followed by injecting TSCs or exosomes into the Achilles tendon. The healing and degradation of the tendon matrix were then evaluated at 5 weeks after the injury using histology analysis and biomechanical testing. In vitro, TSCs treated with interleukin 1 beta were subjected to conditioned medium with or without exosomes or exosomes alone, and tendon matrix-related markers and tenogenesis-related markers were measured using immunostaining and western blotting. The results showed that both TSCs and exosomes injections significantly reduced the expression of matrix metalloproteinases (MMP)-3, increased the expression of tissue inhibitor of metalloproteinase-3 (TIMP-3) and Col-1a1, and improved the biomechanical properties of the ultimate stress and maximum loading. In vitro, conditioned medium with exosomes and exosomes alone also had a similar effect on decreasing MMP-3 expression and increasing the expression of tenomodulin, Col-1a1, and TIMP-3. These findings suggest that exosomes derived from TSCs may be a promising therapeutic strategy for tendon injury healing due to their ability to balance the tendon extracellular matrix and promote the tenogenesis of TSCs.40
Knee treatments
Various nanoparticle systems, such as dendrimers, liposomes, micelles, and exosomes, hold great promise for repairing knee cartilage. Nanoparticles possess the unique ability to persist in cartilage cells for extended periods, allowing them to quickly reach injured cells following direct joint injection. If administered soon after joint injury, nanoparticles may even preserve cartilage viability and avert the onset of osteoarthritis. For rheumatoid arthritis, polyanionic phosphorus dendrimers can be administered intravenously or orally to selectively target monocytes, reduce inflammation, and prevent dendritic cell maturation. Liposomes, which are safe and can be modified to evade clearance by the reticuloendothelial system, are commonly used to treat inflammatory osteoarthritis. Micelles, such as adeno-associated virus, have been employed to boost extracellular matrix components, heal cartilage, prevent erosion, and enhance chondrocyte proliferation. Exosomes are utilized as a core therapeutic agent to repair knee tendons, with their capacity to secrete a range of bioactive factors making them an attractive option for various diseases and injuries.16–38
Liposomes have been established as a safe and effective vehicle for delivering a wide range of drug molecules for the treatment of cartilage injury. These lipid-based nanoparticles can be engineered to evade clearance by the reticuloendothelial system, resulting in enhanced therapeutic efficacy. Lipotalon, a liposomal drug delivery formulation available commercially in Germany, has shown promise in treating inflammatory osteoarthritis, with optimal results achieved through the use of liposomes prepared in precise ratios with phosphate-buffered saline (PBS).41 Similarly, micelles offer a viable option for cartilage repair, with polymeric micelles being utilized as carriers to promote the formation of extracellular matrix components, prevent cartilage erosion, and stimulate chondrocyte proliferation.38 These targeted and controlled micelles represent a promising therapy for repairing or reconstructing articular chondrocytes. Finally, exosomes have emerged as a promising therapy for repairing tendons in the knee. These extracellular vesicles serve as a core agent for resolving the therapeutic effects of multiple factors secreted by mesenchymal stem cells, offering a potential solution for various diseases and injuries.38 A summary of several studies involving nanoparticle based therapies for knee injuries is presented in Table 2.
|
Carrier type |
Drug/Material |
Animal model |
Results |
Reference |
|
Polymeric |
Kartogenin/ PEG- |
Rat |
Better histological and OARSI score at 12 weeks compared to control |
|
|
Nanoparticles |
HDI-N-BOC Serinol |
|||
|
Betamethasone/ PLGA |
Rat |
Decrease in inflammatory cells after 7 days |
||
|
Betamethasone/ PLGA |
Rabbit |
Decreased joint swelling for 21 d |
||
|
Indomethacin/Self assembling PGLA |
Rat |
↓ diameter; favorable histology; ↓ TNFα in serum |
||
|
Curcumin/ Acidactivable PAE |
Rat |
↓ TNF-α and IL1β production, favorable histology |
||
|
Diclofenac- |
Rat |
↓ of OARSI score |
||
|
Kartogenin/Pluronic |
||||
|
Piroxicam/ PLGA + Eudragit RL |
Rat |
Prolonged retention into joint compared to |
||
|
NPs without |
||||
|
Eudragit RL |
||||
|
Etoricoxib/ |
Rat |
Favorable μCT; ↓ |
||
|
PLGA&PEG |
MMP-13 and ADAMTS-5; ↑ collagen and aggrecan |
|||
|
Adenosine/ PLGAPEG |
Rat |
↓ OARSI score |
||
|
Celecoxib/PEAs |
Sheep |
↓ joint effusion; |
||
|
↓ WBC |
||||
|
HA/PLGA |
Rat |
NP are still in the knee after 35 days |
||
|
Berberine chloride/Chitosan |
Rat |
Higher antiapoptosis activity and prolonged i.a. drug retention |
||
|
CrmA/ HA and Chitosan |
Rat |
↓ OARSI score; |
||
|
↓ IL-1β, MMP-3, MMP-13; collagen conserved |
||||
|
Liposomes |
Celecoxib/ Liposome + hyaluronic acid |
Rabbit |
Favorable histology |
|
|
Carbon-based NPs |
KAFAK/Fullerene |
Rabbit |
Favorable histology |
|
|
Hyaluronan conjugation/ Graphene oxide |
Rat |
↓ MMP-3 |
||
|
concentration in the joint |
||||
|
Antisense oligomers/ Carbon nanotubes |
Rat |
Inhibition of protein synthesis in chondrocytes and reduction in inflammation |
||
|
Metallic NPs |
Fish oil protein, both in DPPC liposomes/Gold |
Rat |
↓ IL-1β, IL-12, |
|
|
|
|
|
PGE2, TNF-α |
|
Table 2 Some examples of nanocarriers delivering FDA-approved drugs for treatment of knee injuries [ADAMTS-5, A disintegrin and metalloproteinase with thrombospondin motifs 5 2; CrmA, cytokine response modifier A; DPPC, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; HA, hyaluronic acid; HDI, hexamethylene diisocyanate; IL, interleukin; MMP, matrix metalloproteinase; NGF, nerve growth factor; OARSI, osteoarthritis research society International; PAE, poly(β-amino ester); PEAs, poly(ester amide)s; PEG, poly(ethylene glycol); PLA, poly(lactic acid): PLGA, poly(lactic-co-glycolic acid); pNIPAM, poly(N-isopropylacrylamide); TNF-α, tumor necrosis factor alpha; WBC, white blood cells]
Nanotechnology-based DDS for treatment of alzheimer’s disease
Dementia is a rapidly growing public health concern worldwide, with a new case developing every 3 seconds. The global prevalence of dementia exceeded 55 million in 2020, and this figure is expected to nearly double every 20 years, reaching 78 million by 2030 and 139 million by 2050. The vast majority of this increase is projected to occur in developing countries, with low and middle-income nations already accounting for 60% of people with dementia. This proportion is expected to rise to 71% by 2050.42 Of note, the elderly population is expanding most quickly in China, India, and their neighboring regions in South Asia and the Western Pacific.42 Alzheimer's disease, a neurodegenerative disorder characterized by β-amyloid plaque deposition and neurofibrillary tangles of hyperphosphorylated tau, is currently the most prevalent cause of dementia worldwide. Its incidence is projected to increase due to an aging world population.
The onset of Alzheimer's disease (AD) can occur more than 20 years before clinical symptoms become noticeable, and its progression is gradual and slow.43 Familial AD, which makes up approximately 510% of all AD cases, is linked to specific genes such as Presenilins 1 and 2, alpha-2 Macroglobulins, and Apo-E. In contrast, non-familial or sporadic AD, accounting for roughly 70% of AD cases, is believed to be caused by a combination of genetic, environmental, and lifestyle factors.44 Numerous hypotheses have been put forth to explain the molecular mechanisms underlying Alzheimer's disease (AD) pathogenesis. A thorough understanding of the key factors that are involved in AD neuropathogenesis could lead to the identification of potential therapeutic targets. Possible treatment options for AD include both symptomatic and targeted disease-modifying approaches.45 Symptomatic treatments aim to enhance cognitive and memory functions to improve patients' quality of life. The proposed hypotheses of AD pathogenesis include the cholinergic, amyloid, and tau theories. In addition to these major hypotheses, there is evidence suggesting that reactive oxygen species (ROS), nitric oxide, and inflammatory mediators may also contribute to AD pathogenesis.46
Diagnosis of Alzheimer's disease relies on clinical presentation and various biomarkers. Presently, the treatment of Alzheimer's disease is focused on symptomatic therapy, with ongoing clinical trials aiming to address its underlying pathology.47 An illustration of the various nanocarriers that are used for targeting Alzheimer’s disease in the brain is presented in Figure 4.
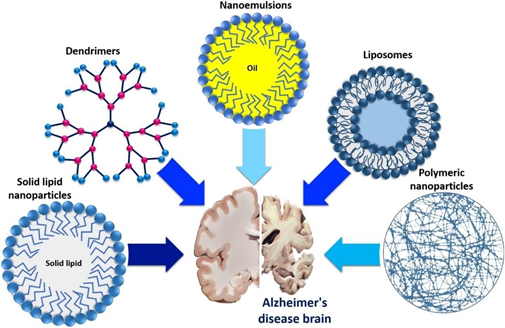
Figure 4 Different promising nanocarriers used for targeting Alzheimer's disease brain.48
Polymeric nanoparticles
Polymeric nanoparticles are colloidal carriers into which drugs can be loaded in either a solid state or as a solution. They are absorbed non-covalently or can be chemically linked to the surface. Their advantages include good stability, biocompatibility, biodegradability, low toxicity and immunogenic response, continuous drug release and ease in manufacturing.49 The mode of administration for polymeric nanoparticles is either orally, subcutaneous, intranasal, or intravenous. The drug transport mechanism can occur via receptor mediated endocytosis or transcytosis of the cells of endothelium, which will result in the release of drugs to the target site.48 It has been reported that PEGylated poly[α,β-(N2-hydroxyethyl)-d,l-aspartamide] (PHEA) nanoparticles, produced through UV irradiation of polymers in an inverse microemulsion, exhibit controlled drug release rates for Rivastigmine in both simulated extracellular fluid and human plasma, indicating that the incorporation method and drug form contribute to improved parenteral administration.50 Poly(lactic-co-glycolic acid) (PLGA) nanoparticles loaded with galantamine were created using a nanoemulsion templating method from oil-in-water (O/W) nanoemulsions.51 The resulting nanoparticles demonstrated high encapsulation efficiency and sustained drug release, preserving the pharmacological activity of Galantamine for intravenous administration. The versatility of this approach has been previously reported, as nanoemulsions can be tailored using the phase inversion composition method to achieve the desired physicochemical properties of the nanoparticles.52 Chitosan nanoparticles coated with polysorbate-80 were formulated and loaded with Rivastigmine using a spontaneous emulsification method for targeted delivery to the brain. When administered intravenously, the prepared nanoparticles increased the concentration of Rivastigmine in the brain of mice by 3.82 times compared to the free drug.53
Lipid nanoparticles
Lipid nanoparticles (NPs) are colloidal dispersions that may serve as an alternative to larger colloidal carriers, such as liposomes, nanoemulsions, and polymeric NPs.54 Depending on their composition, lipid NPs have the potential to exhibit low toxicity and maintain the advantages of other carriers, including controlled drug release, drug targeting, drug protection against degradation, and ease of manufacturing at scale.55 There are two generations of lipid NPs: solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs).56 SLNs consist of a solid lipid core that remains solid at both room and body temperature. In contrast, NLCs are composed of a mixture of solid and liquid lipids to overcome some of the limitations of SLNs, such as poor drug loading ability and poor long-term stability caused by polymorphic transitions of lipids to more stable forms. However, the incorporation of drugs into both SLNs and NLCs is influenced by factors such as the drug's lipophilic nature, lipid type, surfactants used, and production technique.55
The warm oil-in-water (O/W) microemulsion method has been utilized to prepare solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) that encapsulate ferulic acid. Intravenous administration of these nanoparticles resulted in increased protective activity against the oxidative stress induced by Alzheimer's disease (AD) in neurons, indicating the effectiveness of these systems in enhancing bioavailability. It has been reported that lipid nanoparticles can cross the blood-brain barrier (BBB) via endocytotic mechanisms and accumulate in the central nervous system (CNS) due to their lipophilic nature. Furthermore, their small size enables intravenous injection, thereby avoiding uptake by macrophages of the mononuclear phagocyte system.57 A micro-emulsification method was used to create solid lipid nanoparticles (SLNs) loaded with galantamine hydrobromide. This formulation exhibited a significant ability to restore memory in rats with cognitive deficits after oral administration and provided 100% bioavailability enhancement compared to the plain drug. Thus, the SLN formulation shows potential for safe and effective delivery of Galantamine. The study provides preclinical data with desirable outcomes in animal models, indicating the potential promise of this approach in clinical studies.58 SLNs prepared using homogenization-ultrasonication and optimized using simulation software were investigated to find the effect of independent factors on the particle size (PS) and polydispersity index (PDI). TEM and AFM results showed that the SLNs were roughly spherical and had maximum entrapment efficiency, which remained stable over 60 days. In vitro drug release experiments showed that both medications were steadily released from the SLNs. Additionally, in vitro cell-based assays demonstrated the increased safety and effectiveness of the SLNs. Based on the positive results obtained from various in vitro characterizations of SLNs, it was concluded that they can be used as a useful carrier for delivering drugs across the blood-brain barrier (BBB).59
Liposomes
Liposomes are vesicles made of a bilayer of phospholipids that form a spherical shape around an inner aqueous core. Their sizes can range from 50 nm to 100 μm and their surface charge and structure can vary depending on the type of phospholipids used and the manufacturing process. Modified liposomes, such as transfersomes, ethosomes, and phytosomes, can also be created by altering their composition.60–63 Liposomes are considered safe and compatible with biological systems because they are made of phospholipids.60–64 They can encapsulate both hydrophilic and lipophilic drugs, protecting them from degradation and improving their therapeutic efficacy.65 Liposomes have been extensively studied as carriers for improving treatment for Alzheimer's disease. While liposomes are highly lipophilic and can potentially target the brain, the exact mechanisms of blood-brain barrier (BBB) penetration are not fully understood.66,67 Small liposomes can be transported through the BBB via the endocytic pathway, while larger liposomes may not be able to pass through leaky capillaries in the brain.68 Additionally, the charge and type of phospholipid can affect the distribution and stability of liposomes. Negatively charged liposomes are thought to be removed from circulation more quickly than neutral or positively charged liposomes.69 Researchers have attempted to modify liposomal structures to improve their penetration across biological membranes and into their target organs.70
Researchers have developed a nasal drug delivery composite nanosystem for Alzheimer's disease (AD) treatment. They used chitosan and sodium alginate as the carrier polymers and loaded them with the drug Rivastigmine, which is used to treat AD. The composite nanosystem was then characterized for particle size, zeta potential, drug loading, and in vitro drug release. The results showed that the composite nanosystem had a particle size of 198 nm and a positive zeta potential of +14 mV. This positive charge can help the composite nanosystem to adhere to the negatively charged nasal mucosa, thereby facilitating drug delivery to the brain. Furthermore, the positive charge can also prevent the aggregation of particles and increase the stability of the system. The drug loading efficiency was found to be 89%, and the in vitro release study showed that the nanosystem could release the drug in a sustained manner over a period of 8 hours.71 In a study on multifunctional liposomes, liposomes were loaded with mAPO and phosphatidic acid (PA). PA has a high affinity for binding to Aβ, while mAPO enhances the crossing of the blood-brain barrier. The combination of mAPO and PA led to the disaggregation of Aβ fibrils in vitro, showing a synergistic effect.72 Apolipoprotein E2 loaded liposomes have also been studied for their therapeutic benefits in AD. The liposomes targeted brain tissue and showed treatment benefits in vitro using mice AD brains administered with systemic liposomes enveloping therapeutic genes.73 In another study, intravenous injection with surface-modified (transferrin-penetratin) liposomes effectively delivered the therapeutic agent past the BBB, resulting in increased levels of ApoE in the mice brains, showing potential treatment for AD.74
Dendrimers
Dendrimers are a type of polymeric nanoparticles with a unique structure that sets them apart from classical polymers. These large, 3D molecules consist of a core, repeating units, and functional groups such as COONa, COOH, and NH2.75There are different types of dendrimers, including polyamidoamine (PAMAM), carbosilane, poly-l-lysine (PLL), and polypropylene-imine (PPI), which vary based on their core and branches. Through the synthesis process, dendrimers can be controlled in terms of shape, size, polydispersity, and surface structure (hydrophilic/lipophilic, charged/neutral) at the nanoscale level.76 Polyamidoamine (PAMAM) dendrimers are the most widely used type and are suitable for a range of applications, including drug and gene delivery systems and regenerative medicine. They have an inner alkyl-diamine core and an outer shell containing amine branches, and their highly controllable design makes them promising carriers for biomedical applications and effective delivery systems for hydrophobic and insoluble drugs.77–79
The use of intranasal dendrimer preparations presents a cost-effective and non-invasive approach for delivering therapeutic agents. According to Win-Shwe et al.80 administering a single dose of PAMAM dendrimers (3 or 15 µg per mouse) via intranasal delivery to 8-week-old BALB/c mice resulted in an upregulation of BDNF mRNA in the cerebral cortex. The study suggests that the PAMAM dendrimers entered the brain via systemic circulation or olfactory nerve pathways, resulting in altered gene expression.80 Katare et al.,81investigated the potential for PAMAM dendrimers with amine surface groups for delivering haloperidol to the brain via intranasal (IN) and intraperitoneal (IP) administration. Haloperidol, a classic antipsychotic drug, has limited water solubility, which prevents its administration via IN or IP without solubility enhancement or nanocarrier formulation. The drug is also known to cause catalepsy and motor suppression in rats, which can be used as behavioral confirmation of successful brain delivery. The researchers used dendrimer entrapment to increase the solubility of haloperidol by more than 100-fold and evaluated the resulting formulation to determine its potential for targeting waterinsoluble drugs to the brain via IN administration. The release of over 60% of the haloperidol content within 3 hours in 20 mM PBS and within 1 hour in 0.1 N HCl in vitro suggests that the dissolution rate and solubility of haloperidol in the release media were limiting factors. It is expected that the drug will be released more quickly under in vivo conditions where sink conditions are encountered. Formulation D-HP exhibited a similar binding potency to the dopamine D2 receptor as control haloperidol, indicating that the haloperidol was either released easily from the formulation or that the dendrimer-bound drug was able to interact with the receptors.81
Clinical need and challenges for alzheimer’s disease treatment
The current treatment for AD is mainly based on FDA-approved medicines such as Galantamine, Donepezil, Rivastigmine, and Memantine. Etanercept, a tumor necrosis factor blocker, is also prescribed off-label for AD. Tacrine was discontinued in the US in 2013 due to its clinically apparent hepatotoxicity. These drugs are primarily administered orally, with dosage forms including tablets, capsules, solutions, and orally disintegrating tablets. Rivastigmine is also available as a transdermal patch for extended release, and Etanercept is given as subcutaneous injections. Despite ongoing clinical trials of new drugs such as E2609 (Eisai Inc.), which is to be administered orally, efficient transport of these and other medicines through the BBB remains a bottleneck in pharmaceutical research. With few delivery systems available on the market, there is an urgent clinical need and challenge to improve drug delivery. Table 3 summarizes some relevant nanocarriers for managing AD.48
|
Carrier type |
Drug |
Carrier material |
Route of administration |
Reference |
|
Polymeric |
Donepezil |
Chitosan |
Intranasal |
|
|
Nanoparticles |
PLGA (Polysorbate 80-coated) |
Intravenous |
||
|
PLGA-b-PEG |
Intravenous |
|||
|
Galantamine |
Chitosan |
Intranasal |
||
|
PGLA |
Intravenous |
|||
|
Rivastigmine HCl |
Chitosan |
Intranasal |
||
|
Rivastigmine Tartrate |
PLGA, PBCA |
Intravenous |
||
|
Chitosan (Polysorbate 80coated) |
Intravenous |
|||
|
Solid Lipid |
Galantamine |
Glycerylbehnate (Compritol) |
Oral |
|
|
Nanoparticles |
Lipoyl– |
Stearic acid |
Oral |
|
|
Memantine |
||||
|
Rivastigmine HCl |
Compritol 888 ATO |
Intranasal |
||
|
Liposomes |
Rivastigmine HCl |
Phosphatidylcholine; |
Subcutaneous |
|
|
Dihexadecyl phosphate; cholesterol; glycerol |
||||
|
|
Donepezil |
Carboxymethyl cellulose, 1,2distearyl-sn-glycero3-phosphocholine, cholesterol, PEG |
Intranasal |
|
|
Cell penetrating peptide (CPP)modified liposomes |
Rivastigmine HCl |
EPC, cholesterol, |
Intranasal |
|
|
DSPE-PEG-CPP |
||||
|
Flexible liposomes |
Galantamine |
Soya phosphatidylcholine, cholesterol, and propylene glycol as edge activator |
Intranasal |
|
Table 3 Some examples of nanocarriers delivering FDA-approved drugs for Alzheimer's disease. [DSPE, 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine; EPC, Egg phosphatidylcholine; PEG, polyethylene glycol; PBCA, polybutyl cyano acrylate; PLGA, poly lactide-co-glycolic acid]
To date, there are no clinical trials that are investigating the use of nanocarriers for AD treatment. Therefore, the focus should be on the feasibility of translating this approach from the laboratory to clinical practice. The clinical adoption of nanocarriers may face challenges related to their industrial impact and high costs, but their potential benefits, such as their ability to cross the BBB, non-invasive nature, and potential for use in diagnosis and treatment, should not be overlooked. In fact, some studies suggest that these multifunctional systems could enhance the efficacy of drugs. The main challenges to bringing nanocarriers from the laboratory to the clinic are simplifying their preparation methods and finding costeffective approaches for scaling up production.48
The introduction of nanotechnology is poised to revolutionize the drug delivery industry, impacting all administration methods, from oral to injectable approaches. This should result in reduced drug toxicity, lower treatment costs, improved bioavailability, and an extension of the lifespan of proprietary drugs for both doctors, patients, and the healthcare industry. The goal is to maximize solubility and increase drug bioavailability. Nanosuspension technology can benefit class II (high permeability, low solubility), class III (low permeability, high solubility), and class IV (low permeability, low solubility) drugs. By formulating poorly soluble and low bioavailable drugs into nanosuspensions, they can be rescued from abandonment and improved for safety and efficacy. Nanoparticles offer targeted and controlled release, making them a promising drug delivery system. In the future, drugs may intentionally be made insoluble to take advantage of nanosuspension technology. However, significant obstacles must be overcome to make this field a clinically viable therapy. Scientists predict that manipulating material properties at the nanoscale could modify and enhance existing technologies, leaving plenty of room at the bottom. By applying true nanotechnology principles to designing novel delivery systems, drug delivery can benefit further. With sufficient time and research, the promise of nanotechnology-based medicine may become a reality. The future of nanosystems is promising and full of possibilities.
The authors acknowledge with thanks the financial support of the Ravindra Family.
None.

©2023 Ravindra, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.