eISSN: 2574-9927


Research Article Volume 8 Issue 3
Department of Physics, New Jersey Institute of Technology, USA
Correspondence: Nuggehalli M Ravindra, Department of Physics, New Jersey Institute of Technology, Newark, New Jersey 07102, USA, Tel (973) 5963278, Fax 9735965794
Received: August 12, 2024 | Published: September 24, 2024
Citation: Hossain S, Bahreini B, Pasteur E, et al. Magnetotactic bacteria and magnetosomes – an overview. Material Sci & Eng. 2024;8(3):83-100. DOI: 10.15406/mseij.2024.08.00241
Magnetotactic bacteria are a diverse group of prokaryotes that create intracellular, membrane-bound organelles containing magnetic minerals known as magnetosomes. These minerals have a specialized purpose with the use of mineral crystals such as magnetite (Fe3O4) or greigite (Fe3S4). Due to the stability of these crystal structures, the bacteria can orient themselves with the Earth’s geomagnetic field lines, functioning as compass needles to help them locate suitable environments to inhabit. The objective of this review, based on previous progressions in this area of study, is focused on presenting a comprehensive summary of MTB, its abundance and magnetosome formation. Moreover, the effects of certain environmental factors, as well as their significance and applications in material science, medicine, and biotechnology, are elucidated.
Keywords: magnetotactic bacteria, magnetosomes, magnetite, greigite, environmental factors, morphology, physiology, biomineralization, targeted drug delivery, cancer therapy, hyperthermia, bioremediation
In recent years, there has been a significant interest in the study of magnetic field effects on biological systems, particularly in relation to magnetosensation— the ability of living organisms to sense the Earth’s geomagnetic fields for navigation purposes. Presently, there are three accepted main theories that address this interesting phenomenon. For example, one hypothesis suggests that some migratory birds may rely on microscopic magnetic deposits in their beak for orientation. However, this idea remains a subject of debate among researchers, as definitive evidence is still lacking.1 Another intriguing theory posits that certain light-sensitive proteins, known as cryptochromes, are found in the eyes of selective animals and might act as a chemical detector of the Earth’s magnetic field. This idea has gained substantial traction in recent years, but like the magnetic deposits’ hypothesis, it also awaits further experimental validation. An interesting alternative theory for magnetosensation revolves around magnetotactic bacteria (MTB), which are microorganisms that orient themselves along the geomagnetic field lines. The magnetic-sensing hypothesis suggests that these bacteria, living in a symbiotic relation with animals, may serve as the underlying mechanism behind their magnetic sense."2,3 This theory proposes that MTB is the answer to the long-standing enigma of magnetosensation.
Despite the widespread interest and extensive research surrounding these three primary hypotheses, they all share a significant challenge: they are exceptionally difficult to verify. Unlike other sensory stimuli, magnetic receptors, if they exist, could be incredibly small, scattered across extensive areas of tissue, and positioned anywhere within an animal’s body. This makes them exceedingly elusive and hard to detect, posing substantial obstacles to scientific study and experimental validation. The complexity of locating and identifying such receptors means that proving any of these hypotheses requires innovative approaches and advanced technologies that are only beginning to emerge. Consequently, the study of magnetosensation remains one of the most intriguing yet challenging frontiers in sensory biology, with potential applications across various fields, including but not limited to biomedicine, bioremediation, and energy generation.
The potential of MTB and their magnetosomes in the medical and environmental fields are gaining increasing attention. For example, in medicine, MTB could be utilized for targeted drug delivery, improved medical imaging, and magnetic hyperthermic therapy, which serves as a treatment for cancer. Additionally, MTB plays an important role in the natural biogeochemical cycle of sulfur, phosphorus, carbon, iron, and nitrogen. This makes them promising candidates for bioremediation, where they can be employed to clean up pollutants and heavy metals. Moreover, the MTB and magnetosome applications expand to other fields including chemistry, physics, geology, crystallography, food science, and biotechnology.4
Magnetotactic bacteria are a group of Gram-negative motile prokaryotes, and they are particularly interesting because, despite the traditional view that bacteria lack subcellular compartments, it is now understood that they can possess organelles as eukaryotic cells do. MTB for instance, are even capable of biomineralizing membrane-enclosed magnetic nanoparticles inside their cytoplasm".5 These nanoparticles, called magnetosomes, form the MTB’s internal magnetic compass that enables their characteristic ability known as magnetotaxis. This process occurs when the magnetosomes align in sequential order, forming a chain that allows MTB to align with the Earth’s geomagnetic field lines.
The purpose of this review is to present a comprehensive understanding of the current stage of knowledge of MTB, including their characteristics, as well as their applications in accompanying fields. Overall, this paper highlights the available literature on these organisms and the way they can be used to advance science and technology across multiple disciplines.
Magnetotactic bacteria
Magnetotactic bacteria (MTB) are a diverse group of motile prokaryotes that vary morphologically, physiologically, and phylogenetically but share a few key characteristics:
(1) They possess the unique capability of magnetotaxis;
(2) They can synthesize iron oxide nanoparticles composed of magnetite (Fe3O4) and/or greigite (Fe3S4) in different quantities, that when enclosed by a biological membrane forms a magnetosome;
(3) They have flagella that enables their motility; and
(4) They have a microaerophilic and/or anaerobic physiology. The primary function of the magnetosome is to help MTB locate and maintain their optimal position in the environment. MTB are abundant in natural waters, including sediments and chemically stratified water columns.6
The first known occurrence of MTB was observed by Salvatore Bellini in 1963 from water collected from various freshwater environments near Pavia, Italy. He detected large numbers of bacteria swimming in a consistent, single, northward direction and speculated that the magnetic behavior of the cells was due to an internal “magnetic compass.” Richard P. Blakemore, though rediscovering this independently in 1974, was able to validate Bellini’s “magnetic compass” claims within cells of MTB12.
Understanding the origins of MTB is closely related to the biomineralization process, more specifically magnetosome synthesis. Insights into this process and iron regulation capabilities encoded within their genomes are crucial. Genetic analysis of fossilized magnetosomes (magnetofossils) may help uncover the unknown factors that initially allowed MTB to synthesize magnetosomes. In addition, magnetofossils can provide key information about the environmental conditions required for MTB survival.
Magnetofossils have been identified in sedimentary rocks from various ages (Figure 1). They mostly have been found in sediment from lakes, carbonate platforms, pelagic or hemi-pelagic environments.7 Well persevered chains of magnetosomes, about 85 million years (Ma) old, were discovered in Late Cretaceous chalks from Southern England and are thought to be the earliest occurrence during the geologic eon Phanerozoic (0–540 Ma). Magnetofossils from the Precambrian era are rare; however, some terrestrial evidence of their presence has been identified in the 2.0 Ga-old Gunflint Formation and the Tumbiana Formation in Western Australia.
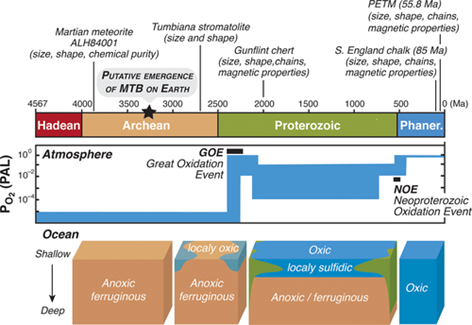
Figure 1 Main geological eons on Earth's history, evolution of atmospheric oxygen, ocean chemistry and occurrences of magnetofossils in the deep time. Oxygen partial pressure expressed in percent of present atmospheric level (PAL).8
There are over 50 species of MTB that have been discovered in various natural environmental conditions that belong to the Alphaproteobacterial, Deltaproteobacterial, Gammaproteobacterial, Nitrospirae, and Omnitrophica bacterial strains.8 Their morphology includes a diverse distribution, featuring shapes such as cocci (sphere), bacillus (short and long rods), vibrio (curved rod), spirilla (twisted) (Figure 2), and multicellular morphotypes9 (Figure 3). All MTB synthesize their own magnetosomes and possess a flagellum. Most single-celled MTB only produce iron oxide (Fe3O4) magnetosomes, while others can produce only greigite (Fe3S4). Multicellular MTB can synthesize both, iron oxide and greigite magnetosomes.9,10
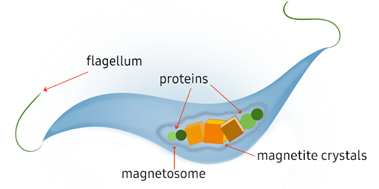
Figure 2 Graphical representation of typical spirillum MTB. Figure made by authors.10
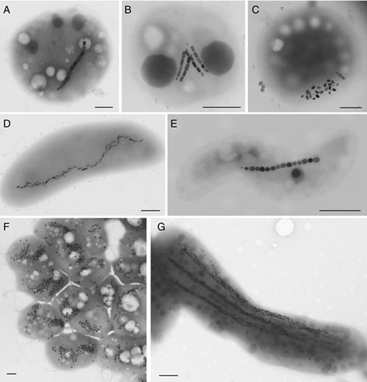
Figure 3 Transmission electron microscopy images of A-C) Magnetotactic Cocci, D) Vibrio, E) Spirillum F), disaggregated Multicellular Magnetic Prokaryote (MMP), and G), Ca. Magnetobacterium bavaricum; B,C,E,G) isolated from various freshwater, and A,D,F) marine environments; Magnetotactic cells in (A–E and G) produce elongated prismatic (A–C and E) or bullet-shaped (D and G) magnetite magnetosomes, while the MMP (F) produce octahedral greigite magnetosomes. Scale bars represent 0.5 μm. (Mattiew Amor, et. Al).9
Magnetotactic bacteria exhibit unique physiological traits that distinguish them from other prokaryotic cells, including the possession of intracellular organelles that form the magnetosomes. On average, MTB contain 10 to 50 aligned magnetosomes composed of iron oxide or greigite magnetic nanoparticles and proteins.9,10 These, along with their flagella, are key to their ability to perform magnetotaxis. Magnetosomes are typically organized in a chain sequence, allowing the bacteria to align with the geomagnetic field lines while it moves.
Magnetotaxis can be classified as polar, or axial.10 Axial magnetotactic cells are enabled to swim in both directions within the geomagnetic field lines, while polar cells are either moving towards the North Pole (north seeking), or towards the South Pole (south seeking). In other words, axial MTB uses the geomagnetic field lines as an axis of motility, while polar MTB use them for motility and direction.9–11
From a phylogenetic perspective, most identified MTB belong to the phyla Protobacteria, Nitrospirae, and candidate phyla Omnitrophica and Latescibacteria.11 Researchers have hypothesized that gene evolution involved in magnetosome formation could reveal the transformation of magnetotaxis".4,12–15 Despite the phylogenetic difference, all MTB possess unique genes related to biomineralization, located in an unstable region of the genome.16 For example, in M. Gryphiswaldense strain MSR-1 and other freshwater spirilla, the genes are grouped into four operons within the magnetosome island: mamAB, mamGFDC, mms6, and mamXY.17 While some MTB species have different sizes in the biomineralization region, there are other genes that are conserved and preserved throughout all species, specifically the mamAB gene.
MTB are known to produce minerals of iron oxide and iron sulfide18 and are present in both marine and lake environments. Magnetotactic bacteria of varying morphological types have been found in freshwater sediments. Most of which flourish in sediments or chemically stratified water columns, but predominantly occur at the oxic-anoxic interface (OAI), the anoxic regions of the habitat, or both.19 Generally, magnetite-producing MTB are found at or very close to the OAI, while greigite producers are present in reducing biotopes, below the OAI, in the sulfidic anoxic zone. A wide variety of MTB have been found in salt marshes, particularly in the surface layers of sulfidic sediments where they co-occur with sulfide oxidizing bacteria.19 Consequently, MTB are excellent examples of gradient (e.g., oxygen concentration and redox)-loving organisms.
As mentioned in the introduction, one hypothesis of magnetosensation suggests that MTB live in symbiosis with animals that possess magnetic reception abilities. However, identifying these magnetoreceptors is extremely difficult, requiring the use of various techniques such as superconducting quantum interference device (SQUID) magnetometry, X-ray fluorescence, and atomic force microscopy, among others. Currently, evidence of magnetic receptors in animals has been found in pigeons and trout as showcased in Figure 5. In pigeons, a sophisticated sequence of six clusters of magnetic minerals has been found at the beak coupled to a nerve that reacts to magnetic stimuli. The system, which is in the branches of nerve cells, consists of a 3-5μm diameter vesicle coated with a non-crystalline iron compound and surrounded by ten to fifteen 1μm spherical clusters. Each of these contains around 8 million 5nm diameter crystals of magnetite and maghemite chains consisting of ten 1x1x0.1μm size plates.20 The theory is that these receptors interact with MTB to facilitate magnetoreception and magnetosensation. Ferromagnetic and ferrimagnetic minerals can interact with magnetic fields, enabling compass mechanism caused by spontaneous ordering of electron spins.
MTB are distributed worldwide, thriving mainly in aquatic ecosystems. A common objection to the ecological significance of MTB is their perceived rarity, which suggests that they are not regularly involved in specific symbiotic relationships.3 However, it is important to emphasize that they are generally abundant in sediments and chemically stratified water columns of various aquatic environments, e.g. freshwater, brackish, and marine environments such as lagoons, estuaries, lakes, rivers, hydrothermal vent, seamounts.21 MTBs commonly inhabit the environments, as described above in large numbers, especially at the OAI of sediments of water and as they are mostly microaerophiles.21
Recently, specific types of MTB were discovered in unusual and extreme environments. For example, C. Nash identified the presence of thermophilic magnetotactic bacteria in microbial mats at about 45 to 55°C adjacent to the main flow in Little Hot Creek, and noticed their absence in other springs in the same area at 40 to 80°C. Nash also identified MTB in microbial mats of other springs in central California at up to 58°C as well as all on the east side of the Sierra Nevada Mountain Little Hot Creek (45–55°C) and four from Mono Lake (pH of 9.8 and salinity 80.8 gL−1).18 This marked the initial indication that some MTB can thrive in extreme environments including hot springs, saline-alkaline lakes and deep-sea environments.22 Microbial magnetoreception, i.e., the ability to use magnetic fields for orientation, navigation, and homing12 has only been observed in aquatic microorganisms so far and seem intrinsically linked to magnetotaxis.23 Other ecological strategies that make use of these schemes include predation of magnetotactic bacteria and symbiosis with magnetic bacteria (Figure 4). Further studies of MTB in these and other environments could provide important information and opportunities to address questions regarding the origin and evolution of magnetotaxis and biomineralization in bacteria. There appears to be no reason why other extremophiles, including acidophilic, piezophilic, halophilic, or psychrophilic bacteria, could not have acquired the ability to biomineralize magnetosomes.12
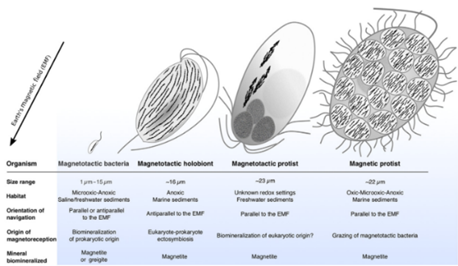
Figure 4 Schematic comparison of Magnetoreceptive Microorganism Morphologies and Magnetic Behaviors. Magnetosome chains are represented in black. The organism’s anterior-posterior orientation is given relative to the Earth’s magnetic field (EMF) direction in the Northern Hemisphere. These orientations are the reverse for microorganisms of the Southern Hemisphere (Christopher T. Lefèvre et. Al).15
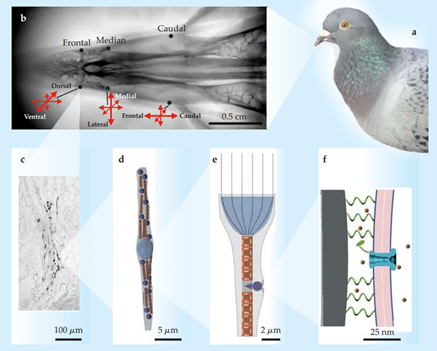
Figure 5 Evidence for magnetite-based magnetoreception:
(a) The homing pigeon Columba livia provides some of the best evidence.
(b) X-ray image of the upper beak of C. livia, showing the three pairs of iron-containing areas and the prevailing orientations of their neurons.
(c) Stained section of the dendritic region in one of the areas. Dark areas are iron deposits.
(d) Schematic of a single neuron, showing the centrally located, iron-coated vesicle (light blue) and the clusters of magnetite crystals (dark blue) alternating with rows of maghemite plates (red).
(e) Hypothesized concentration of magnetic flux in a neuron and its effect on the position of one of the magnetite clusters.
(f) Magnetite cluster pulling away from a membrane, which bends and opens a mechanically stimulated ion channel. Panel a courtesy of Andreas Trepte; panels b–c adapted from G. Fleissner et al., Naturwissenchaften 94, 631, 2007; panels d–e adapted from G. Fleissner et al., J. Ornithol. 148, 643, 2007; panel f adapted from I. Solov’yov, W. Greiner, Biophys J. 93, 1493,2007.21
Understanding the linkage between the abundance and diversity of MTB, as well as their environmental temperature is of great interest for researchers. Still, properly assessing this relationship through direct field studies would be challenging due to the high variability of environmental factors. For that reason, microcosm studies offer a solution by eliminating external factors and placing microorganisms in a controlled environment. For instance, in the process of biomineralization, specifically in biologically controlled mineralization (BCM), minerals are usually deposited on or within the cell. During the BMC process, the organism exerts a significant degree of control over the nucleation and growth of minerals influencing the overall size, composition, habit, and intracellular location. Therefore, this process is highly controlled at both the metabolic and genetic level.4
Cultivation
The detection of MTB in samples collected from natural environments is relatively straightforward in comparison to the isolation and axenic cultivation of MTB in pure culture, which is much more challenging.19 Despite these organisms being discovered over six decades ago, only a small number of isolates are available; the population of isolates in pure culture become even smaller when considering species that biomineralize magnetite, and there are no isolates for greigite-producing strains.24 The isolation process is set back even further by MTB’ physiology as extension nutritional requirements in diverse species. Additionally, MTB are clearly gradient-requiring organisms. The oxygen/redox gradient is difficult to replicate in growth medium in laboratory12. Moreover, iron is a requirement for magnetosome synthesis and thus must be present in the growth medium. Nonetheless, iron source is not as critical if the MTB is kept at a neutral pH in the presence of reducing agents. For example, the growth of cultivated species of Magnetospirillium is inhibited by iron concentrations > 200 μM. This implies that biomineralization of magnetite is not unique to iron rich environments. Composition and concentration of salts in growth medium are important for marine strains of MTB. The salts help to avoid osmotic stress when isolating MTB from saline environments; the medium should be diluted to the salinity of the sample. Alternatively, filtered water from the sample could be used to make the medium.19 Generally, the slow growing MTB are usually outgrown by other non magnetotactic microorganisms in pure culture. Thus, a common strategy used to cultivate them is to inoculate the enrichment medium with purified MTB through a capillary racetrack. Water containing the MTB is placed on a sterile wet cotton plug in the wide end of Pasteur pipette filled with water from the original source. The south pole of the bar magnet is placed near the sealed tip of the capillary furthest from the reservoir. Migration to and accumulation of MTB cells can be observed at the tip of the pipette which can then be broken off and removed using a syringe needle and transferred to an enrichment media.22 A secondary method to isolate MTB in pure culture is by using agar plates of appropriate media as many typical agars may contain impurities. Since activated charcoal is known to decompose toxic free oxygen radicals that inhibit the growth of microaerophiles the plates, it is considered an appropriate agar media.19
Magnetosome formation
Bacterial magnetosome offer numerous advantages compared with the chemically synthesized magnetite, including consistent shape and narrow grain size distribution. Magnetosomes are made up of magnetic mineral crystals, such as magnetite (Fe3O4) or greigite (Fe3S4) and depending on which type of mineral dominates, MTB can change in size, shape, and overall crystal structure. MTB can absorb up to 2-3% iron by dry weight, and in certain microhabitats, they make up a greater portion of the microbial biomass (up to 30%) and play a dominant ecological role in the sediment layers. Previous research has concluded that MTB may contribute about 1-10% of the iron flux in chemically stratified environments, and therefore MTB has an important function in the aquatic iron cycle.25
MTB biomineralization is the process that produces the magnetic mineral crystals within magnetosomes. This process is highly valued amongst researchers as it is believed that it can provide the answers to the magnetotaxis phenomena. Moreover, this process a highly controlled, which is regulated by genetic and biochemical pathways with key genes such as mam and mms families. These genes aid MTB to form crystal chains of magnetite and/or greigite depending on the bacteria strain.20
Magnetosome synthesis is a complex, yet controlled, process that is carried out through various crucial steps. These include vesicle formation, generally about 3-4 nm in thickness in Magnetospirillium species; iron cell absorption; iron transportation; and biomineralization. The transportation of iron into the membrane vesicles is a particularly fundamental step. Scanning Electron Microscopy (SEM) shows that magnetite crystals increase in size even after the membrane vesicle has fully enveloped them. Although it is unclear which redox form of iron is transported into the magnetosome vesicles, researchers have studied this process in strain AMB-1 vesicles. Generally, post-synthesis, the space between each magnetosomes is about 9.0 nm; however, this can vary as it heavily depends on MTB cultivation and the environmental conditions (Figure 6). Overall, these processes are highly controlled by biomolecular machinery, which are made up of many different proteins that can only be found within the membrane vesicles of the MTB".26–29
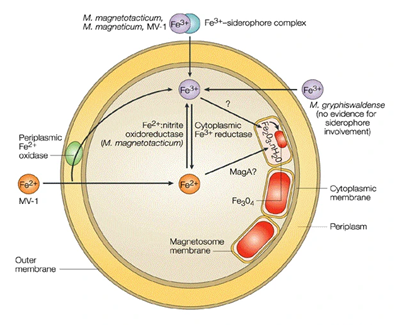
Figure 6 Schematic of probable reactions leading up to magnetite biomineralization in cultured species of cocci magnetotactic bacteria (Bazylinski, D. et. al).30
Magnetosome synthesis varies in morphology depending on the MTB strain that is producing it. Most of the known MTB produces magnetite (Fe3O4) crystals, but there are strains known to produce greigite (Fe3S4) structures. The crystal size, structure, orientation, and arrangement of magnetosome are highly relevant for the magnetic properties of the bacteria.30 Table 1 provides a summary of the various types of crystals formed by the different strains:
|
Strain |
Magnetosome mineral |
Phylogenetic affiliation |
Habit |
Magnetosome elongation axis |
Avg. length (nm) |
Width/ length |
References |
|
Magnetospirillum magnetotacticum strain MS-1 |
Magnetite |
Alpha-proteobacteria |
Cuboctahedral |
** |
43 |
0.9 |
Devouard et al., 1998; Buseck et al., 2001; Kobayashi et al., 2006 |
|
Magnetospirillum magneticum strain AMB-1 |
Magnetite |
Alpha-proteobacteria |
Cuboctahedral |
** |
45 |
0.85 |
Li et al., 2009 |
|
Magnetospirillum gryphiswaldense strain MSR-1 |
Magnetite |
Alpha-proteobacteria |
Cuboctahedral |
** |
33 |
0.91 |
Scheffel et al., 2006; Faivre et al., 2008 |
|
Magnetospira thiophila strain MMS-1 (MV-4) |
Magnetite |
Alpha-proteobacteria |
Elongated, Octahedral |
[111] |
22–85 |
0.85 |
Meldrum et al., 1993a; Devouard et al., 1998 |
|
Magnetovibrio blakemorei strain MV-1 |
Magnetite |
Alpha-proteobacteria |
Elongated, Octahedral |
[111] |
60 |
0.65 |
Meldrum et al., 1993a; Devouard et al., 1998; Thomas-Keprta et al., 2001; Clemett et al., 2002 |
|
Magnetovibrio blakemorei strain MV-2 |
Magnetite |
Alpha-proteobacteria |
Elongated, Prismatic |
[111] |
30–59 |
0.54 |
Meldrum et al., 1993a |
|
Magnetococcus marinus strain MC-1 |
Magnetite |
Alpha-proteobacteria |
Octahedral, Elongated |
[111] |
30–110 |
0.93 |
Meldrum et al., 1993b; Devouard et al., 1998 |
|
Strain MC-2 |
Magnetite |
Alpha-proteobacteria |
Octahedral, Elongated |
ND |
30–120 |
0.85 |
Devouard et al., 1998 |
|
Candidatus Magnetococcus yuandaducum strain YDC-1 |
Magnetite |
Alpha-proteobacteria |
Elongated, Prismatic |
ND |
108 |
0.64 |
Lin and Pan, 2009 |
|
Strain MO-1 |
Magnetite |
Alpha-proteobacteria |
Octahedral, Elongated |
ND |
64 |
0.89 |
Lefèvre et al., 2009 |
|
Magnetospira sp. QH-2 |
Magnetite |
Alpha-proteobacteria |
Octahedral, Elongated |
ND |
81 |
0.71 |
Zhu et al., 2010 |
|
Uncultured Coccus Itaipu-I |
Magnetite |
ND |
Elongated, Prismatic |
[111] |
210 |
0.9 |
Lins et al., 2005 |
|
Uncultured Coccus Itaipu-III |
Magnetite |
ND |
Elongated, Prismatic |
[111] |
130 |
0.6 |
Lins et al., 2005 |
|
Uncultured Coccus |
Magnetite |
ND |
Elongated, Prismatic |
[111] |
< 80 |
0.88 |
Simpson et al., 2005 |
|
Uncultured Coccus |
Magnetite |
ND |
Elongated, Prismatic |
ND |
ND |
ND |
Buseck et al., 2001 |
|
Strain BW-2 |
Magnetite |
Gamma-proteobacteria |
Octahedral |
ND |
67 |
0.94 |
Lefèvre et al., 2012 |
|
Strain SS-5 |
Magnetite |
Gamma-proteobacteria |
Octahedral, Elongated |
[111] |
86 |
0.75 |
Lefèvre et al., 2012 |
|
Strain ZZ-1 |
Magnetite |
Delta-proteobacteria |
Elongated, Bullet, dts1 |
ND |
84 |
0.44 |
Lefèvre et al., 2011b |
|
Strain ML-1 |
Magnetite |
Delta-proteobacteria |
Elongated, Bullet, dts1 |
ND |
ND |
ND |
Lefèvre et al., 2011b |
|
Strain AV-1 |
Magnetite |
Delta-proteobacteria |
Elongated, Bullet, dts1 |
[100] |
30–120 |
0.45 |
Lefèvre et al., 2011d |
|
Desulfovibrio magneticus strain RS-1 |
Magnetite |
Delta-proteobacteria |
Elongated, Bullet |
[100] |
40 |
0.5 |
Sakaguchi et al., 1993; Pósfai et al., 2006 |
|
Ca. Desulfamplus magnetomortis strain BW-1 |
Magnetite |
Delta-proteobacteria |
Elongated, Bullet |
ND |
55 |
0.6 |
Lefèvre et al., 2011c |
|
Uncultured Multicellular |
Magnetite |
Delta-proteobacteria |
Elongated, Bullet, dts1 |
[100] |
104 |
0.4 |
Keim et al., 2007 |
|
Ca. Magnetananas tsingtaoensis |
Magnetite |
Delta-proteobacteria |
Elongated, Bullet |
ND |
102 |
0.37 |
Zhou et al., 2012 |
|
Ca. Magnetobacterium bavaricum |
Magnetite |
Nitrospirae |
Elongated, Bullet |
ND |
110–150 |
ND |
Spring et al., 1993 |
|
Strain MHB-1 |
Magnetite |
Nitrospirae |
Elongated, Bullet |
ND |
119 |
0.35 |
Flies et al., 2005 |
|
Strain MYR-1 |
Magnetite |
Nitrospirae |
Elongated, Bullet |
[100] |
104 |
0.36 |
Li et al., 2010 |
|
Strain MWB-1 |
Magnetite |
Nitrospirae |
Elongated, Bullet |
ND |
116 |
0.35 |
Lin et al., 2012 |
|
Ca. Magnetoovum mohavensis strain LO-1 |
Magnetite |
Nitrospirae |
Elongated, Bullet, fts2 |
[110] |
70–200 |
0.36 |
Lefèvre et al., 2011a,d |
|
Ca. Thermomagnetovibrio paiutensis strain HSMV-1 |
Magnetite |
Nitrospirae |
Elongated, Bullet, fts2 |
[110] |
30–220 |
0.45 |
Lefèvre et al., 2010, 2011d |
|
Strain SKK-01 |
Magnetite |
Candidate Division OP3 |
Elongated, Bullet |
ND |
110 |
0.34 |
Kolinko et al., 2012 |
|
Uncultured MMP |
Greigite |
Delta-proteobacteria |
Equidimensional, Irregular; Elongated, Irregular |
** and [100] |
60–90 |
0.86 |
Pósfai et al., 1998a,b |
Table 1 Summary of the main MTB strains and their magnetite crystal structure. Table retrieved from Posfai et al34
Synthetic magnetites usually have different particle size distributions and morphology, especially when compared with MTB’ biosynthesized magnetosomes.31 Synthesized magnetite is particularly investigated because it can substitute the need for MTB cultivation and dependency of their magnetic properties. There are several physical, biological, and chemically available methods for magnetite synthesis. These include co-precipitation methods, thermal decomposition, sol-gel methods, hydrothermal synthesis, microemulsion, to mention a few examples.
Below is a table, Table 2, with magnetite properties:
|
Property |
Value |
|
Molecular formula |
Fe3O4 |
|
Color |
Black |
|
Density |
5.18 g/cm3 |
|
Melting temperature |
1583-1597 °C |
|
Hardness |
5.5 |
|
Type of magnetism |
Ferromagnetic |
|
Curie temperature |
858 K |
|
Saturation of magnetization at 300K |
92-100 Am2/kg |
|
Standard gibbs free energy of formulation |
-1012.6 kJ.mol |
|
Crystallographic system |
Cubic |
|
Structure type |
Inverse Spinel |
|
Lattice parameter |
Å = 0.8396 nm |
Table 2 Summary of main physicochemical properties of magnetite32
A major advantage of MTB synthesized magnetosomes is that they generally possess consistent size and shape, which is highly desirable in biomedical applications, where small particles can reach small capillaries.
Applications
Magnetotactic bacteria and magnetosomes have extensive applications in the medical, biotechnological, environmental and geological fields. These applications include but are not limited to drug delivery, cell separation, food science, DNA and antigen recovery/detection, hyperthermia, MRI image contrast, enzyme immobilization and bioremediation. A comprehensive summary of these applications is provided in Table 2.
The use of MTB in medical and environmental applications presents significant opportunities and challenges. In the medical sector, MTB's potential for targeted drug delivery is limited due to the need for large culture quantities, being able to control their magnetic properties, and achieving biocompatibility within the human body. Guiding MTB precisely to target sites like solid tumors, especially when they are located deep within the body, coupled with the complicated body’s complex environment, can be a concern about their effectiveness and overall short- and long-term safety. On the other hand, in environmental applications, using MTB for bioremediation requires stable conditions for bacterial survival, while also posing risks of unintended ecological disruption if they spread beyond controlled areas. Ethical considerations, including, but not limited to the use of genetically modified organisms, biosafety, and potential environmental impacts, add further complexity to their application. Addressing these challenges will require major advances in technology, rigorous safety testing, and careful regulation to ensure the responsible use of MTB in areas of healthcare and environmental remediation.
Pharmacokinetics and immunogenicity
In the field of biomedical applications, one area of interest is the pharmacokinetics of MTB and its magnetosomes, including their absorption, distribution, metabolism, and elimination, in both veterinary and human use, as well as the immunogenicity. A study by M. Haripriyaa and K. Suthindhiran explores the effects of magnetosomes and overall immunogenicity reactions. This research investigates both in vitro and in vivo applications, using cervical cancer cell lines (HeLa) and BALB/c mice, respectively, as subjects.32
For this study, Magnetospirillum gryphiwaldense (MSR-1) species was cultured in Magnetospirillum growth medium (MSGM) by Hungate anaerobic technique. The pellet was obtained by centrifugation and thereafter the magnetosomes were extracted by sonification process. The magnetosome morphology and size was analyzed through SEM at a magnification of 50 kx at 20 keV in conjunction with Energy-Dispersive X-Ray Spectroscopy (EDS) to measure the intensity and energy distribution generated by the electron beam striking the magnetosomes.33 The results revealed that the extracted magnetosomes weighted on average 5-7 mg/L, presented cubo-octahedral shapes with sizes ranging up to 120 nm, presented iron oxide as well as proteins, lipopolysaccharides, and phospholipids in the magnetosome membrane.34
The in-vitro analysis (Figure 7) assessed the absorption of Magnetosome-FITC (Mag-FITC) in HeLa cells at concentration levels of 200, 150, 100, 50, and 10 µg/mL.
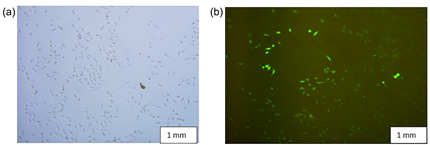
Figure 7 (a) Represents the control image of HeLa cell lines and (b) represents the magnetosome- FITC absorption process in HeLa cell lines recorded at 100mg/mL Mag-FITC concentration.30
The in-vivo analysis (Figure 8) biodistribution of the Mag-FITC using bioimaging system was assessed at various time intervals (5, 30, 60, 90 minutes; 12, 24, 48, and 72 hours).
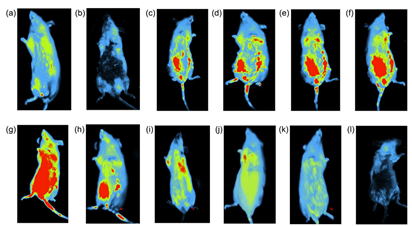
Figure 8 Biodistribution of Mag-FITC assessed at different time intervals (Scale bar: 5.0 cm) (a) control (sodium chloride) (5 min) (b) control (sodium chloride) (2 h) (c) 5 min (d) 30 min (e) 60 min (f) 90 min (g) 12h (h) 24h (i) 36 h (j) 48 h (k) 60 h (l) 72 h.30
This HeLa cell line study revealed a cell uptake of 97% with 200 μg/mL concentration, which suggests their capacity to deliver therapeutics directly into the cells and use magnetism to control their movement within the body. Moreover, the study revealed that total Mag-FITC was completely metabolized from the system after 72 hours (Figure 7).
Controlled and targeted drug delivery (TDD) methods are diligently being researched because they present a possibility to improve drug pharmacokinetics and minimize the release and unwanted side effects to non-target sites. A promising approach to TDD applications involves MTB or magnetosomes, which can accumulate in desired locations with the guidance of an external magnetitic field, light, or ultrasound wave. Among the aforementioned driving mechanisms, the magnetic field is optimal in the sense of biosafety, efficacy, and control range. A major advantage of utilizing iron oxide particles (F3O4) and maghemite (γ-Fe2O3) over other high magnetic moment particles, such as cobalt and nickel, is that they do not cause oxidative stress or long-term enzyme changes.35 Moreover, in the realm of biomedical applications, it is desired to have biodegradable systems to avoid side effects if left within the human body.30–32
The delivery method to specific locations needs to be appropriately small in size, to penetrate specific tissues or vascular capillaries that can be 4-5µm in diameter, as well as hypoxic regions where diffusion is limited due to an absence of flow.36 Simultaneously, systemic toxicity must be minimized while therapeutic index is increased. Therefore, this would require a low-cost, high efficiency multifunction nanorobotic system, which can be challenging to produce with the current technological feasibilities.24 A practical, economical, and naturally available self-replicating system that could fulfill this role involves MTB as an alternative to artificially synthesized agents. Moreover, the ability of MTB to activate key immune cells such as dendritic cells, NK cells, and T cells (Figure 9) offers a promising approach to enhancing the immune response against tumors. By stimulating both the innate and adaptive branches of the immune system, MTB may help improve cancer immunotherapy, enhance the effectiveness of existing treatments, and provide new therapeutic strategies for targeting solid tumors. The combination of MTB's immunostimulatory effects and their targeted delivery capabilities represents a potential breakthrough in the field of cancer treatment. With that said, MTB can potentially reach even low oxygenated tissue given their small size, and coupled with cell or gene therapy therapeutics, the immunological response can be enhanced and target the cancerous tissue more effectively.
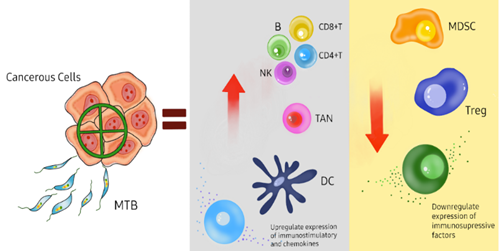
Figure 9 Schematic representation of how magnetotactic bacteria (MTB) can be used to target cancerous cells for therapeutic purposes. The MTB, along with cancer therapies can specifically target solid tumor cells, leading to the upregulation of immunostimulatory cells such as dendritic cells (DC), natural killer cells (NK), T cells (CD8+ and CD4+), and B cells, as well as tumor-associated neutrophils (TAN). This activation enhances the immune response to the tumor. Concurrently, cancer therapies downregulate the production of myeloid-derived suppressor cells (MDSC) and regulatory T cells (Treg), as well as other immunosuppressive factors, thereby inhibiting the tumor's ability to evade the immune system. Figure made by Authors.41
In the oncology space, TDD is particularly attractive for the treatment of solid tumors as the risk of invasive interventions and metastasis could be significantly minimized or eliminated during treatment in early stages.37 The targeted delivery of drug-carrying MTB could be injected directly onto the solid tumor, and utilizing an external magnetic field, hyperthermic conditions can be created to raise the temperature to 43-47°C and destroy the tumor cells.
In 2016, Felfoul et al. used Magnetococcus marinus (MC-1) strain to transport drug loaded nanoliposomes37 to oxygen depleted hypoxic regions of solid tumors in the colorectal region of A/J and C57BL6 mice. The results showed a significant improvement in the therapeutic index of nanocarriers when attached to MTBs.37 The loaded bacterial cells were injected near the site of interest and magnetically guided to it, showing marked penetration into the interior of the tumors where most active cancer cells reside compared to passive agents. The bacteria were alive and motile, and exhibited magnetoaerotaxis responses despite being marine organisms. They were not expected to live within mammalian systems and the mice displayed no negative effects from being exposed to these bacteria. This is unusual as gram negative bacteria generally induce an immunological response within living systems and these MTBs were considered “clinically safe” in this study. However, further studies need to be conducted on the effect of introducing MTBs into living systems to guarantee safety. MTBs do not multiply, cause infection, or severe immunologic responses because they lack the endotoxic lipopolysaccharide that is present in the outer membranes of Gram-negative cell walls.38 Figure 10 displays some advantageous and disadvantageous properties of MTBs and magnetosomes that make them eligible candidates for drug-carrier designs.39
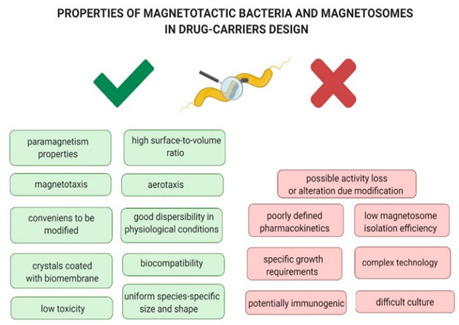
Figure 10 Properties of Magnetotactic bacteria and magnetosomes as advantages and disadvantages in drug-carriers design (Kuzajewska, D et. al).41
Bioremediation
MTB and their magnetosomes can be doped with various metals to enhance their functionality for bioremediation (Figure 11) and biomineralization processes. By incorporating transition metals other than iron into the magnetosomes, the magnetic properties of the magnetite crystals are altered, enabling the absorption and immobilization of these metals. This modification allows for the magnetic removal of MTBs from sediments and water. This capability can be leveraged to design custom magnetic biomaterials with specific, targeted functions".38–42 Shimoshige et al. studied the effects of the transition metal samarium on the formation and properties of magnetosomes in Magnetospirillum magneticum strain RSS-1. It was found that the synthesis of magnetosomes was elevated in the presence of a combination of samarium and iron and each core magnetic crystal structure formed was covered in a layer of samarium oxide. This finding suggests the possibilities of magnetic recovery of transition metals and the formation of novel magnetic structures and particles that can be used to develop eco-friendly nanomaterial synthetic technology.43 In 2017, Zhou et al successfully genetically engineered Magnetospirillum gryphiswaldense to improve removal of phosphorus from wastewater and recovered the bacteria using magnetic fields. Other studies have doped magnetosomes with Cu, Mn, and Co in various Magnetospirillum species".46–48 Tanaka et al48 observed tellurium uptake and crystallization within Magnetospirillum magneticum but as tellurite concentrations increased in the growth medium, a decrease in magnetotactic response among the cells was noted. Thus, a limiting factor of using MTBs for bioremediation is the excessive presence of compounds or metals of interest might hamper the magnetotactic response inevitably making the removal of these cells difficult.22
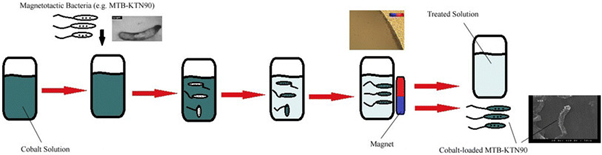
Figure 11 Schematic representation of magnetic separation of metal-loaded MTB (e.g., MTB-KTN90) for the bioremediation processes (Tajer-Mohammad-Ghazvini et. al).49
Energy generation
Faraday's law of electromagnetic induction states that a current will be induced in a circuit or conductor when it is exposed to a varying magnetic field, with the induced current being proportional to the rate of change of the magnetic flux intersecting the circuit. The magnetic properties of magnetotactic bacteria (MTBs) can be harnessed for electricity generation. Smit et al. successfully demonstrated this concept by developing a method to use the biomineralized magnetic nanoparticles of MTBs as a biological alternative for generating low voltage electricity (Figure 12).47 Here, an alternating current was observed and measured, and its presence is attributed to several factors. For example, the magnetic nanoparticles passing through the solenoid acts as separate magnetosomes or magnetosome strings rather than being uniformly distributed, consistent with Faraday's observations. In this experiment, a net voltage (measured in millivolts) was detected, with significantly higher values in purified magnetosomes compared to MTB culture and liquid medium growth. The voltage was directly proportional to the magnetosome concentrations until an optimal concentration of 0.0376 mg/ml was reached. Although the generated voltage was very small, this method holds potential for applications where alternative energy sources are needed, particularly in cases where low voltages are relevant. Further investigations are required to optimize the setup to produce economical amounts of energy.37,49
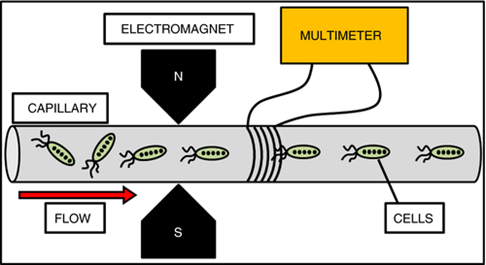
Figure 12 Schematic representation of the experimental setup in which the voltage produced by different materials was tested. The suspensions were pumped from left to right in the capillary and magnetic materials were aligned by the electromagnet if it was needed. Materials then passed through the solenoid and induced an emf on the coil. This voltage was then measured by the multimeter and analyzed.52
Drug delivery and novel therapeutic strategies
Magnetic nanoparticles as delivery vectors are of great interest in the field of nanotechnology and biomedicine as they have the potential to resolve drug administration issues such as biodistribution, specificity, affinity, concentration, and toxicity (Figure 13 & 14). Bacterial Magnetosomes (BMs) are considered ideal nanocarrier candidates as they possess unique characteristics such as paramagnetic properties, nanoscale, narrow-size distribution, and being bounded to the membrane.50
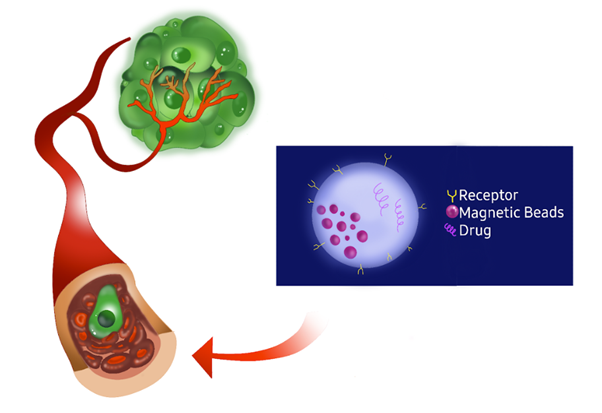
Figure 13 Illustration of targeted drug delivery using magnetic beads. The magnetic beads are conjugated with receptors and drugs, allowing them to specifically target and bind to tumor cells. This targeted approach enables the magnetic beads to concentrate the drug in the tumor site, enhancing the efficacy of the treatment while minimizing the impact on healthy tissues. Figure made by Authors.51
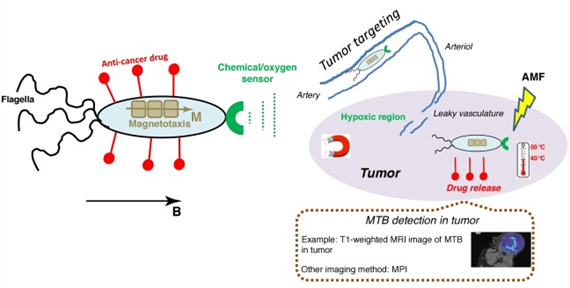
Figure 14 Schematic representation of how whole magnetotactic bacteria (MTB) can target, destroy, or image a tumor. Targeting can be realized using their aerotactism or magnetotactism, enhanced permeability and retention (EPR) effect, or magnetic targeting; antitumor efficacy can be achieved by MTB carrying and releasing chemotherapeutic drugs in the tumor or MTB producing heat under alternating magnetic field (AMF) application; and imaging of MTB can be performed using magnetic resonance imaging (MRI) or magnetic particle imaging (MPI). The tumor part is indicated by the violet ellipsis. Bacteria are located in tissues external to the tumor before they enter it.66
Cancer therapy
Sun et al loaded chemotherapy medication, Doxorubicin, onto purified BMs to create a complex that was examined against the HL60 and EMT-6 cell lines of human leukemia and mouse breast cancer to determine its antitumor activity.21 It was found that the complex did not degrade significantly and 80% of the drug remained bound until it reached its target tissue without any loss in its antitumor activity.50 Other cancer therapeutics such as daunorubicin, cytarabine and epriubicin have also been immobilized onto magnetosomes successfully".29,51–54 Deng et al found that cytosine arabinoside (Ara-C) conjugates demonstrated high stability and strongly enhanced controlled drug release over the period of almost 3 months thus mitigating severe side effects of the drug.55
Hyperthermia
The hyperthermia cancer treatment involves raising the temperature of the target tumor to 43-56 degrees Celsius, which increases its sensitivity to radio- and chemotherapy, and stimulates the immunological system response. The mechanism of action takes place under a magnetic field, where the magnetic energy is converted into thermal energy, which destroys targeted tissues. The carriers are also reinforced with binding agents such as antibodies, hormones, and exo- or endogenous substances. Present implementations for tumor targeting rely on an external magnet, usually restricted to areas near the skin, lowering the efficacy as the target tissue is deeper in the human body.56
Hyperthermia alters the function of many critical structural and enzymatic proteins within cells thus disrupting essential cell processes like cell growth, differentiation, etc. and triggering cell apoptosis. It has immense potential to act as an alternative cancer therapy to be used either solely or in conjunction with chemotherapy or radiotherapy. Current therapeutic systems that employ hyperthermia have no toxic side effects which make them appealing; however, they are limited by their inability to target specific sites and the resultant tissue temperature distribution. These issues could be addressed using magnetically mediated hyperthermia (MMH) which consists of localizing magnetic particles in tumor tissue and then application of an external alternating magnetic current that causes them to heat by hysteresis loss, Neel relaxation or induced eddy current.57 Synthetic magnetic nanoparticles are not very effective as they lack specificity and selectivity but magnetosomes could be an excellent alternative to them as they can be designed with reagents to target specific zones".58–64 An alternating magnetic field can also be used to control the release of drugs from functionalized magnetosomes.29,65
Conjugates with antibodies
The objective of immunotherapy is to activate the host’s immune response to cancer cells. It is an emerging field of study with many successful strategies involving immunocompetent drugs. Cancer cells can evade the immune system’s defensive response allowing them to grow tumors. They can change their appearance and suppress genes that could act as markers for T lymphocytes to recognize them. This can be countered using immunopharmacologic drugs that induce apoptosis in malignant cells such as 4-1BB antigen (CD137)—an inducible co-stimulatory receptor found on the surface of T lymphocytes and natural killer (NK) cells.29,66 Tang et al. functionalized magnetosomes complexes with an agonist antibody against 4-1BB which encouraged the proliferation of murine T lymphocytes and secretion of tumor necrosis factor α (TNF-α) and interferon γ (IFN-γ).29,67 These findings were confirmed in vivo by intravenous administration of the conjugates in mice with lung cancer and directing them to the tumor site with magnetic fields [AA].68,69 The conjugates also displayed superior infiltration into tumor tissue and higher recruitment of tumor-infiltrating lymphocytes (TILs) in comparison to free form antibodies.70 The permeability of magnetosome-antibody conjugates in tumor tissue was also investigated by Erdal et al. using anti-epidermal growth factor receptor (EGFR) antibody and it was found that they penetrate tumor tissue to a higher degree than iron oxide nanoparticles coated with the same antibody.68
Carriers of vaccine DNA
Attaching tumor antigens or fragments to magnetosomes to enhance the immune system’s response to cancer cells is another immunotherapeutic strategy employed by scientists to combat malignant growths. Xiang et al. developed novel complexes of magnetosome- polyethylenimine (PEI) electrostatically bound to DNA with improved transfections in vitro and in vivo when exposed to magnetic fields.69 The vaccine potential of this strategy was also determined in mice immunization tests with magnetosomes-PEI system in conjunction with a plasmid encoding the VP1 protein from the envelope of the hand, foot, and oral disease (HFMD) virus. The results demonstrated increased production of anti-VP1 antibodies and significantly higher activation of T lymphocytes with the application of a magnetic field [G, CC]. Further studies have been conducted using similar strategies to confirm the vaccine potential of this application.70–72
Gene therapy
Gene therapies involve alternating genes to treat or prevent diseases by either replacing faulty genes, inactivating malfunctioning ones, or introducing new genes, often using viral vectors or CRISP-Cas9. However, the most common gene delivery methods into the cell are associated with oncogenesis and inflammation. MB are alternative delivery methods and can be inserted into the cells resulting in the expression of iron storage proteins that can be detected via MRI.73 This delivery system has been studied using modified cationic chitosan-coated iron oxide nanoparticles.74
Altering oncogenes and tumor suppressor genes in malignant cells can disrupt and inhibit cellular functions that lead to metastasis.75 This can be achieved using developing technologies such as gene therapy. Introducing therapeutic elements into target regions is one the main hurdles to overcome when considering this technology but a solution to this issue could be by utilizing magnetosomes as the delivery vector. Han et al developed a targeted gene delivery system by creating a complex containing Polyamidoamine (PAMAM) dendrimer and Tat peptides with magnetosomes along with plasmids that downregulate EGFR gene in human glioblastoma U251-MG cells. This resulted in the inhibition of rate of glioma cell proliferation in vitro, induction of apoptosis and loss of permeability to the laminin- and collagen-containing matrix barrier.76 These results were confirmed successfully in mouse models as well. Dai et al used siRNA with Polyethyleneimine (PEI) and magnetosome to create a conjugate that was tested on HeLa cell line. Results showed reduced RNA degradation due to immobilization on the magnetosomes and cell apoptosis along with proliferation inhibition among the target cells.77
Drug co-delivery systems
One method of developing co-delivery platforms that can precisely transport two or more therapeutics in one system is the use of magnetosome complexes. An efficient co-delivery system using siRNA and doxorubicin loaded magnetosome-PEI conjugates was developed by Long et al. In vitro tests results indicated sustained release of doxorubicin, reduction in RNA degradation, and synergistic efficacy of inhibition of proliferation among target cells.78 Other exciting results have been achieved by Cheng et al with a heat guided system involving a combination of doxorubicin-magnetosome conjugate with a recombinant plasmid that codes for heat shock protein promoter 70 (HSP70). Their effects were evaluated on osteosarcoma, and it was determined that the constructs generated enough heat to induce hyperthermal conditions that had antitumor effects. This system, illustrated in Figure 15, has potential application in combined chemotherapy, gene therapy, and thermotherapy for osteosarcoma.79
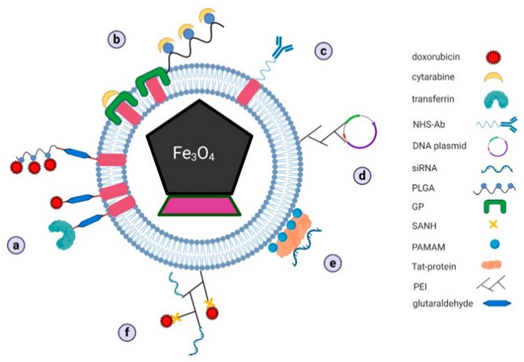
Figure 15 Schematic representation of proposed mechanisms of drug docking on bacterial magnetosomes for: (a) doxorubicin, (b) cytarabine, (c) antibodies, (d) vaccine DNA plasmids, (e) siRNA, and (f) complex of two different drugs in combined therapy (GP–genipin, NHS–N-hydroxysuccinimidyl, PAMAM–polyamidoamine dendrimers, PEI–polyethyleneimine, PLGA–poly-L-glutamic acid, SANH–succinimidyl 6-hydrazinonicotinate acetone hydrazone). [30, Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license].
Cell separation
Magnetosomes have been introduced to leucocytes via phagocytosis and magnetic fields were used to successfully concentrate and separate them from samples containing cells that had not ingested the particles".29,80 Reconstructed magnetosomes expressing antibody-linking protein have also been successfully used to separate and recover peripheral blood cells (Monocytes and B-lymphocytes) that were pretreated with anti-CD14, CD19 and CD20 murine monoclonal antibodies [MM]. Takahashi and group genetically engineered multifunctional magnetic nanoparticles to express characteristics of polyethylene glycol (PEG) to enhance their recognition of and binding to target cells which were then magnetically separated [NN].81,82
Food safety
Immunomagnetic separation (IMS) is a technique used to efficiently isolate and concentrate target bacteria from food samples and magnetosomes are ideal candidates for this purpose due to their many favorable characteristics.83 Various studies have used magnetosomes based complexes to isolate and capture pathogens such as Vibrio parahaemolyticus, Salmonella dublin, Staphylococcus aureus enterotoxin, etc".84–86
DNA and antigen recovery/detection assays
Magnetosomes have been chemically functionalized as reagents for protein detection assays by Ceyhan and group. DNA oligonucleotides were bound into the magnetosomes which allowed them to bind to complementary DNA with specificity. This technology is significant as it has potential applications in bio analytics, imaging, and other fields of nanobiotechnology. Antibody-functionalized magnetosomes conjugates have been used to immobilize and magnetically enhance the signal generating detection complex in Magneto Immuno-PCR (M-IPCR), thus enabling the establishment of a surface independent immunoassay.87
Magnetic resonance imaging (MRI) contrast agents
Magnetic nanoparticles are particularly useful for MRI because they disrupt the local magnetic field, which creates a net field inhomogeneity that can be captured as a negative contrast using MRI.
Developing more efficient medical imaging methods is possible using magnetically labeled cells that can be monitored using MRI technology. This can aid in the understanding of cellular immigration and pathophysiology of diseases given the resulting strong contrast these agents provide. The potential of anionic magnetic nanoparticles (AMNPs), ultrasmall paramagnetic iron oxides (USPIOs), and superparamagnetic iron oxide nanoparticles (SPIONs) for such applications is very significant.
Magnetosomes were successfully employed as superparamagnetic contrast agents for MRI by Herborn et al. in 2003.88,89 Consequently, they have been used to image various cell models such as pancreatic cells, brain cells, cells of xenograft tumors and breast cancer cells".29,90–93
Enzyme immobilization
Magnetosomes can be functionalized to express catalytic units due to their protein display system and thus are ideal candidates for support of enzyme immobilization.29 Moreover, they have advantageous characteristics over competing materials such as higher surface area which allows for greater enzyme loading, lower mass transfer resistance, less fouling effect, and selective, nonchemical separation from the reaction mixture by an applied magnetic field.94 Ginet et al. genetically fused genes from Flavobacterium sp. that code for a phosphohydrolase on to mamC proteins on magnetosome membranes.95 The functionalized magnetosomes showed sustained enzymatic activity after purification suggesting long term stability and this was confirmed by using them to degrade pesticides repeatedly. Honda et al constructed a multi-enzyme complex on the magnetosome membranes and tested cellulose degradation activity. It was determined that the complexes retained 70% of cellulose degradation activity even after 5 cycles. This method has potential uses in the production of biofuels.29,96
Figure 16 presents a schematic representation of a functionalized magnetosome according to each application described in the Biotechnology section of this review.
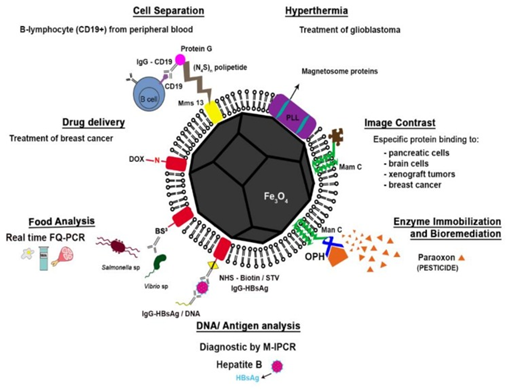
Figure 16 Schematic representation of a functionalized magnetosome according to each application described in the Biotechnology section of this review (cell separation; hyperthermia, drug delivery, image contrast, food analysis, enzyme immobilization and bioremediation and DNA and antigen recovery/detection). Drug delivery: the association between the surface proteins of the magnetosome and doxorubicin (DOX), an anti-breast cancer Cell separation: the modified magnetosomes were bound to anti-murinic G Ig anti-CD19 and used for separating B-lymphocytes from peripheral blood cells. Food sciences: a capture system with the magnetosome proteins fused using a cross-linking reagent bis(sulfosuccinimidyl) suberate (BS3) for attachment of antibodies to Salmonella and Vibrio species from food samples (e.g., milk, egg and pork) DNA/Antigen analysis: antibody-functionalized magnetosomes were used for immobilization of HBsAg (hepatitis B antigen) in human serum and enhancement of sensitivity of immunoassay Image contrast: magnetosomes with specific proteins bound to the surface with high affinity to target cells were used as superparamagnetic contrast agents for magnetic resonance imaging Hyperthermia: magnetosomes coated with poly-l-lysine (PLL) were used in hyperthermia Enzyme immobilization and bioremediation: magnetosome expressing MamC fused with organophosphohydrolase (OPD) from of Flavobacterium sp., were used for the degradation of paraoxon.42
Table 3 presents a summary of the biotechnological applications of whole magnetotactic bacteria (MTB) cells and magnetosomes
|
A) Applications of whole MTB |
||||
|
Field |
Application |
Ref. |
Advantages |
Disadvantages |
|
Drug delivery |
Drug-loaded nanoliposomes attached to Mc. marinus cells for targeted tumor treatment |
Dispenses cell lysis; Uses cell’s own magnetotaxis |
Potentially immunogenic due to outer LPS |
|
|
Bioremediation |
Wastewater treatment; Removal of heavy metals (Cd, Te, Se) |
47–50 |
Magnetic crystal doping possible; Recovery of removed minerals |
Poor growth of MTB in contaminated media; Biomineralization may be affected |
|
Energy generation |
Electricity generation by cells and magnetosomes of Ms. magneticum AMB-1 by means of electromagnetic induction |
Green energy technology |
Only millivolts generated; Expensive |
|
|
B) Applications of magnetosomes |
||||
|
Field |
Application |
Ref. |
Functionalization method |
Advantages |
|
Drug delivery |
Delivery of antitumor drugs: doxorubicin, cytarabine, daunorubicin; delivery of gangliosides; Antitumor gene delivery |
54–83 |
Chemical crosslinking with glutaraldehyde and genipin/PLGA; Surface adsorption of plasmids |
Targeted drug delivery; Reduction of drug toxicity; Tissue specificity; Easy functionalization |
|
Cell separation |
Sorting of blood cells; |
Binding protein expression by vector cloning; Insertion of modified binding protein into membrane |
Reutilization of capture complex; High specificity separation |
|
|
Food safety |
Capture of Salmonella and Vibrio cells; Enterotoxin detection |
87–90 |
Crosslinking of antibodies |
Reutilization of capture complex; High sensitivity |
|
MRI contrast agent |
Diagnostic detection of tumors |
93–97 |
No functionalization; Chemical coupling of targeting peptide |
May also be used as therapeutic tool (by hyperthermia, drug delivery); High affinity to target cells; High detection sensitivity |
|
DNA/Antigen Recovery/Detection |
Capture of oligonucleotides and antibodies; Hepatitis B antigen detection |
Biotinylation by chemical crosslinking with NHS |
High sensitivity and recovery efficiency |
|
|
Hyperthermia |
Treatment of tumors |
60–65 |
No functionalization, generally |
Less significant side-effects than chemotherapy and radiotherapy; Tissue specificity; May also be used as diagnostic tool |
|
Enzyme immobilization |
Bioremediation of organophosphate pesticides; Cellulose degradation |
98–100 |
Enzyme expression by vector cloning |
Reutilization of nanobiocatalyst; Immobilization of multiple catalysts |
Table 3 Summary of biotechnological applications of whole magnetotactic bacteria (MTB) cells (A) and magnetosomes comparing advantages and limitations of each approach (B). [30, Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/)]
List of patents
A list of some relevant patents and patent applications is presented in Section V – Table 4.
|
Active |
||
|
Application No. WO2024095238A11 Year: 2023 Institution: Superbrewed Food Inc. |
|
Title: Magnetosomes comprising a doped mineral and methods of production thereof. Abstract: A magnetosome with a doped mineral is presented, along with a method for large-scale production. This method involves continuous fermentation of magnetotactic bacteria for at least 100 hours, supplying the fermentation broth with iron ions, a dopant, and selected nutrients, adding oxygen, and removing part of the broth containing the bacteria at specified rates. |
|
Application No. US-202202576712 Year: 2022 Institution: Paris Science et Lettres |
|
Title: Magnetic bacteria, non-therapeutic and therapeutic uses thereof. Abstract: Recombinant, metabolically active bacteria include a heterologous prokaryotic biomineralized ferritin. Non-magnetic Escherichia coli can be engineered to become magnetic through the expression and biomineralization of Pyrococcus furiosus ferritin. Magnetic E. coli strains retain their magnetic properties through asymmetrical cell division. These magnetic bacteria have potential uses in both non-therapeutic and therapeutic applications, including biosensing target substances, environmental depollution, displaying antibodies, nanobodies, and antigens, delivering therapeutic substances to target cells, and targeting and infecting target cells. |
|
Application No. US11759478B23 Year: 2021 Institution: Nanobacterie |
|
Title: Non-Pyrogenic preparation comprising nanoparticles synthesized by magnetotactic bacteria for medical or cosmetic applications. Abstract: A non-pyrogenic preparation contains nanoparticles synthesized by magnetotactic bacteria for medical or cosmetic applications. These nanoparticles consist of a crystallized mineral core, primarily iron oxide, surrounded by a coating that excludes material from the magnetotactic bacteria. |
|
Application No. US-11369699-B24 Year: 2020 Institution: Nanobacterie |
|
Title: Magnetic nanoparticles for destroying pathological cells in an individual. Abstract: A composition containing magnetic nanoparticles for treating a tissue volume with pathological cells, where only part of the tissue volume is occupied by the nanoparticles upon administration, and the nanoparticles are activated by radiation. |
|
Application No. CN109251878B5 Year: 2018 Institution: Heilongjiang Bayi Agricultural University. |
|
Title: Thiobacillus ferrooxidans capable of producing magnetosomes with high yield and application thereof. Abstract: The invention pertains to the use of Thiobacillus ferrooxidans for high-yield magnetosome production. The strain, preserved as CCTCC NO: M2018630, is easy to culture with a growth period of 24-36 hours, producing 6.28 mg of magnetosomes per gram of biomass. This strain serves as a biological factory for magnetosome production, applicable in drug delivery and other uses. |
|
Application No. US10238886B26 Year: 2017 Institution: Nanobacterie
|
|
Title: Treatment of cancer or tumors induced by the release of heat Generated by various chains of magnetosomes extracted from magnetotactic bacteria and submitted to an alternating magnetic field. Abstract: A method for treating tumors, tumor cells, or cancer in a subject involves generating heat using chains of magnetosomes extracted from magnetotactic bacteria and exposed to an alternating magnetic field. These magnetosome chains exhibit strong antitumoral activity, whereas unbound magnetosomes or those within whole bacteria do not. Adding chelating agents or transition metals to the bacteria's growth medium enhances the heating properties of the magnetosome chains. Additionally, encapsulating the magnetosome chains in lipid vesicles can improve their rotational and heating capacity in vivo, potentially releasing an antitumoral agent within tumors when heated. |
|
Application No. US10993905B27 Year: 2013 Institution: Natura Bisse International Sa. |
|
Title: Cosmetic compositions comprising magnetosomes and uses thereof. Abstract: The present invention relates to cosmetic and skin care compositions comprising magnetosomes. The invention also relates to methods for the treatment of diseases associated with increased proliferation or accumulation of differentiating keratinocytes. |
|
Application No. CN103449533B8 Year: 2017 Institution: Suzhou lihewen Technology Co., Ltd. |
|
Title: A kind of magnetotactic bacteria sampling culture instrument. Abstract: The invention provides a method for restoring soil polluted by heavy metals using a combination of magnetotactic bacteria and plants. Magnetotactic bacteria are mixed with contaminated soil, irrigated, and exposed to a magnetic field to collect the contaminated soil with bacteria. The soil is then eluted and returned. Bamboo willow and He Hong Chard dishes are planted in the contaminated soil, with the chard removed and replanted every 50-60 days. This process is repeated over 1.5 to 2.5 years until the heavy metal content in the soil reaches safe levels. The method offers advantages like easy collection of bacteria, a short growth cycle for bamboo willow, high metal uptake, and effective cadmium enrichment by He Hong Chard dishes, without secondary pollution to the soil. |
|
Pending |
||
|
Application No. CN105193733A9 Year: 2014 Institution: Northeast Forestry University. |
|
Title: Medicine slow-release nanoparticle wrapping magnetosomes of magnetotactic bacteria and preparation method of medicine slow-release nanoparticle. Abstract: The invention relates to a medicine slow-release nanoparticle that encapsulates magnetosomes from magnetotactic bacteria, along with a method for preparing these nanoparticles. The nanoparticles are composed of magnetosomes, chitosan, a pharmaceutical adjuvant, and medicinal components. The preparation process involves collecting magnetosomes, mixing them with the other materials, and using spray drying to create nanoparticles, which range in size from 100 nanometers to 50 micrometers. These nanoparticles can be used as an oral slow-release formulation or for targeted medicine, making them widely applicable in the field of biological medicine.
|
|
Withdraw And/ Or Abandoned |
||
|
Application No. US20100135912A110 Year: 2009 Institution: Leland Stanford Junior University |
|
Title: Magnetotactic bacteria MRI positive contrast enhancement agent and methods of use. Abstract: Magnetic resonance imaging (MRI) is enhanced by contrast agents like superparamagnetic iron-oxide (SPIO) particles, which resemble magnetite particles produced by magnetotactic bacteria. Magnetospirillum magneticum AMB-1 generates ultrasmall magnetite particles (10-40 nm) that provide positive MRI contrast, allowing for clearer image distinction compared to negative contrast. Studies showed that these bacteria increased positive MRI contrast 2.2-fold in vitro and 2.0-fold following intratumoral injection in mouse tumor xenografts. Upon intravenous delivery, M. magneticum AMB-1 targeted tumors, enhancing MRI contrast 1.4-fold. Tumor targeting was confirmed via viable counts, microPET imaging, and Prussian blue staining, demonstrating the potential of magnetotactic bacteria for improving cancer diagnosis and treatment monitoring through MRI. |
|
Application No. US20090311194A111 Year: 2006 Institution: Emory University Georgia Tech Research Corp |
|
Title: Methods and compositions for the production and use of magnetosomes Abstract: Methods and compositions are provided for using magnetosomes as cellular contrast agents and markers for magnetic resonance imaging (MRI). These methods include synthesizing magnetosomes in cells via a nucleotide construct containing an exogenous polynucleotide sequence. The magnetosomes act as contrast agents or markers for MRI. The invention also includes methods for synthesizing and isolating magnetosomes for introduction into immune-matched cells within tissues or subjects. Additionally, methods for stably transfecting cells to express a polypeptide that modulates magnetosome production are provided, along with the cells produced by these methods, transgenic animals containing such cells, and vectors and delivery systems for cell transfection. Furthermore, the invention includes non-invasive methods for generating visible images of tissues or subjects containing transfected cells, monitoring cell location, migration, or proliferation, and detecting or monitoring gene expression. |
Table 4 Summary of MTB related 1) Active; 2) Pending; 3) Withdraw and/or abandoned patents
Magnetosomes have many advantages that make them ideal candidates for applications in a variety of fields. These include but are not limited to 1) uniform particle shape and size; 2) high chemical purity; 3) unique magnetic properties (they are single magnetic domains); 4) an apparent low toxicity; and 5) the possibility of bioengineering and functionalization due to the magnetosome membrane [G]. Advances in molecular biology and other fields have allowed for more efficient and cost-effective protocols and technologies to culture, purify and functionalize magnetotactic bacteria and magnetosomes. This has improved their use in fields such as biomedicine, bioremediation, imaging, etc. As the use of MTBs and magnetosomes gain popularity in the scientific world, more research needs to be done to address their impact on health. They could be tested on different cell lineages to determine how they are processed by the cells and if the method of administration affects these processes. Heavy metal uptake and metal processing is another element to consider to fully realize their potential and impact in bioremediation. Overall, MTBs and magnetosomes are promising tools that have significant impact on applied biotechnology, nanotechnology, and medicine.97–116
None.
None.
The authors declare that there is no conflict of interest.

©2024 Hossain, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.