eISSN: 2574-9927


Research Article Volume 5 Issue 2
1Air Pollution Research Department, Environmental Research Division, National Research Centre, Egypt
2Refractories, Ceramics & Building Materials Department, Inorganic Chemical Industries and Mineral Resources Research division, National Research Centre, Egypt
Correspondence: Atef MF Mohammed, Air Pollution Research Department, Environmental Research Division, National Research Centre, Giza, Tel +201151143456, Fax +20 2 33370931
Received: April 06, 2021 | Published: April 23, 2021
Citation: Saleh IA, El-Hemaly SAS, Mohammed AMF. Impact of air pollutants on some building materials in Cairo atmosphere. Material Sci & Eng. 2021;5(2):49-58. DOI: 10.15406/mseij.2021.05.00156
Damage of materials is one of the most important adverse effects of air pollutants. The present investigation was undertaken to identify the extent of damage caused to some building materials by air pollutants in Cairo atmosphere and to what extent the pollutants accelerate the “natural” corrosion of materials. Samples of six different types of building materials (limestone, sand-lime brick, neat cement samples, cement blocks, industrial gypsum samples and LECA samples) were exposed to the ambient atmosphere for a period of three successive years at five locations, having different loads of air pollution represent residential and industrial areas through Cairo City. The changes in physical and chemical properties of the exposed materials were determined. It was found that the deterioration of building materials, exposed to Cairo city, was high related to the atmospheric pollution load with reference to sulfate, chloride and nitrate concentrations. Higher correlation coefficients bet
ween the compressive strength losses and the atmospheric SO2 and NO2 doses were found for cement block and limestone samples. The mineralogical composition (X-ray diffraction) of the product films of the exposed building materials showed the formation of gypsum and hydrated calcium silicate components in the building materials and cement content.
Keywords: air pollution, gaseous pollutants, particulate matter, deterioration, building materials
Gaseous and particulate air pollutants are significantly affect non-biological materials. Of particular importance are effects on building stones, historic and cultural monuments, which create an important part of our cultural heritage.1–3 The effect of the deposition of atmospheric gases and aerosols on building materials constitutes one of the main damage mechanisms threatening the cultural heritage. Physical changes and chemical interaction occur at the building surface when exposed to outdoor atmosphere. The action of chemicals usually results in irreversible changes. Consequently, chemical damage to materials is of more serious problem.3,4 Dry deposition of gases plays an important role for the deterioration of stone materials. The dry deposition of gaseous air pollutants on stone and other materials is influenced by atmospheric processes and the chemical characteristics of the deposited gas species and of the specific receptor materials.5–7 The absorbed gases may act directly on the material, or first be converted to new substances that are responsible for observed effects. Gaseous pollutants such as sulfur dioxide and nitrogen oxides can react directly with the stone surface by forming acids in the presence of water (or moisture) and oxidizing agents. The formed acids react with the stone to form salts which either crystallize out within the stonework resulting in physical damage or they are washed away resulting in a loss of material.8–10 Sulfurous or sulfuric acids are capable of attacking a wide variety of building materials, including limestone, sandstone, concrete, marble, roofing slate, mortar, etc. Fairly soluble sulfates are formed, which are then leached away by rain.11,12
In Egypt, which is a repository for many buildings of history, museums and monuments, no previously serious study on air pollutants-induced damage on building materials could be traced. The cultural treasures are irreplaceable, so their preservation from the destructive effects of airborne contaminants poses a significant challenge to their present conservators. The aim of this work is to identify the environmental damage caused to some building materials by air pollutants in Cairo atmosphere and to assess to which extent urban and industrial atmosphere affect and accelerate the “natural” corrosion of materials. The study of environmental damage on stones and building materials is of fundamental importance in both the preservation of modern buildings and corrects planning of conservation works on historical monuments, in which such materials are commonly used.
Samples of six different types of building materials were exposed to the outdoor atmosphere at five locations, having different loads of air pollution represent residential and industrial areas throughout Cairo City. The chosen sites were:
The tested building materials
The building materials that were investigated are:
Generally, these stones and building units are widely used in buildings and as constituents of archaeological religious artifacts and historical monuments.
Preparation and exposure of samples to outdoor atmosphere
The stone and building unit samples were cut (or moulded) as cubes of approximate dimensions of (3x 3x3cm) and mounted on wooden tables placed over roofs of buildings in the selected sites. All stone and building unit samples were exposed to the atmosphere for a period of successive three years. Five samples of each type were removed from each site after exposure of one, two and three years interval and were taken off to the laboratory for the measurements.
Mechanical test
Mechanical testing of building stones offers possibilities of measuring the atmospheric deterioration of buildings. Mechanical test was determined from the compressive strength measurements.13,14 Compressive strength measurement was conducted by using a universal-testing machine (VED Werkstoff pruf maschiner Leipzing M/C.S No. 1316062, Fabr. Nr.264/37, Scales 0-60/ 40/ 20 tons). Three samples (of dimensions 3x3x3cm) were tested for each type after all the exposure intervals. Control samples were also examined and the change in compressive strength due to the exposure to the atmosphere at the different chosen locations was recorded. The reported results are the average for three samples and expressed as kg/cm2.
The mineralogical composition of building materials (X-ray Diffraction)
The product films constituents of the investigated building samples, exposed at residential and industrial districts for a period of three years, were examined by X–ray diffraction analysis and compared with the control samples. The product film of the top surface of each type of building samples were ground and packed in a plastic holder; no adhesive or binder was added. X-ray powder patterns were obtained at room temperature using a Philips diffractometer (Type pw 1399) employing Ni-filtered CuK α-radiation (λ=1.5404Å). The X-ray tube was operated at 36Kv and 25mA. The diffraction angle 2θ was scanned at a rate of 2 degrees. min-1.
Air pollution measurements
Atmospheric pollutants in both forms (particulates and gases) were measured with the exposure of materials. Daily (24 hours) measurements of sulfur dioxide, nitrogen dioxide and suspended particulate matter were monitored at the same selected sites in Cairo city. West and Geak method was applied for the determination of sulfur dioxide.15 Nitrogen dioxide was measured according modified Saltzman technique.16 Suspended particulate matters was measured depends on the filtration technique. Vacuum pump with rate of 10liter/minute and membrane filter paper (<10μm) were used for collecting the suspended particulate matters. Deposited particulate matter was also detected at the selected sites. Deposited dust collectors were used for collecting dust deposited from the ambient air of investigated sites. The collectors consist of cylindrical glass beakers 17cm in height and 8 - 9.5cm diameter. The cylindrical glass beaker was half filled with distilled water to avoid re-entrainment of the collected dust and mounted on iron tripods at a height of 1.2m above roof level to avoid the collection of surface dust and placed between at least 2.4–15m above ground level.17,18 Sulfates, chlorides and nitrates were determined in the water-soluble portion of both suspended and deposited particulate matter by using ion chromatography. Statistical analysis of data included determination of arithmetic mean ±S.D. For studying as association between two variables, correlation coefficient (r) was used.19,20
Figures 1A-1F show the average compressive strength of the chosen building units exposed at the various districts in Cairo after successive three years of exposure. The figures clearly indicate that the compressive strength for all the examined building materials were decreased at all the districts compared with the control one. This is mainly due to the external effects and internal weakness in building stones, due to the action of air pollutants, and the formation of imbrittlement crusts at the surfaces of stones. As it has been expected, the lowest deterioration, in turn of compressive strength were recorded for building samples exposed to the atmosphere of the pure residential area with low population and low human activities (site 1). This is not surprising, since from pollution results of this study, the atmosphere of site (1) have a relatively low pollution load compared to those of other districts of Cairo City (Table 1). On the other hand, the highest deterioration was recorded at site 5 (industrial area) for all the examined samples where high concentrations of gaseous and particulate pollutants are emitted due to the industrial activities.

Figure 1 The average compressive strength of building units exposed at the various sites in Cairo City for a period of three years.
|
Pollutants |
Site 1 |
Site 2 |
Site 3 |
Site 4 |
Site 5 |
|
|
SO2(ug/m³) |
21.63 |
71.65 |
46.90 |
22.22 |
120.68 |
|
|
NO2(ug/m³) |
56.52 |
110.63 |
82.75 |
93.98 |
227.21 |
|
|
Deposited |
Sulfates |
17.58 |
24.56 |
26.80 |
37.66 |
77.49 |
|
Chlorides |
3.44 |
6.00 |
6.74 |
15.41 |
22.94 |
|
|
Nitrates |
2.12 |
1.69 |
1.85 |
1.80 |
2.79 |
|
|
Suspended |
Sulfates |
20.02 |
34.97 |
40.17 |
48.86 |
76.60 |
|
Chlorides |
5.26 |
7.11 |
11.54 |
18.68 |
49.74 |
|
|
Nitrates |
5.03 |
4.49 |
5.22 |
5.21 |
8.10 |
|
Table 1 Annual mean concentrations of air pollutants at the different investigated sites
Deposited salts expressed as mg/m2.day; Suspended salts expressed as µg/m3
The lower compressive strength for limestone samples was 193kg/cm2 (exposed at site 5) compared to 313 kg/cm2 for the control samples (Figure 1A). This means that the compressive strength was decreased by about 38.3 % of its original value at this site after three years of exposure (Figure 2). Meanwhile, at site 1 it reached 280 kg/cm2, i.e., it lost 10.5 % of its blank reading. This higher loss in compressive strength at the industrial area of site 5 is mainly due to that this area has received the highest rate of particulates precipitation, especially sulfates, and SO2 concentrations which are highly destructive to limestone. These results are confirmed by21,22,23 who stated that “In the presence of SO2 and acidic compounds, carbonate building stone, calcite (CaCO3), reacts to form CaSO3.2H2O and CaSO4.2H2O (gypsum), which are highly susceptible to surface erosion”. Also, gypsum has a larger volume than calcite, which results in tension and possible fractures.1-3 In Cairo atmosphere due to the lack of rain, gypsum is probably not dissolved and instead it deposited and forms a surface layer on the stone, which may eventually crack and peel off causing the higher loss in compressive strength. The significant deterioration in the strength of samples at site 5 can be also attributed to the presence of high rate of chloride precipitation from the industrial activities which lead also to attacking the building limestone. This is confirmed by the results of.6,24–26 The erosion effects are not limited to the surface, as acidic pollutants can penetrate into the interior through the pore structure of the stone leading to a further decrease in compressive strength.
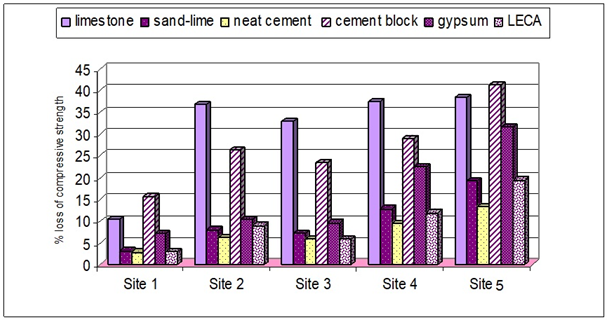
Figure 2 The percentage loss of compressive strength for different building units at various investigated sites in Cairo City after three years of exposure.
In case of sand-lime samples (Figure 1B), compared with the control samples of 22kg/cm2, the compressive strength at site 5 (industrial area) decreased to 181kg/cm2, which represents a loss of 19.2%. The deterioration of sand-lime samples exposed at industrial area is six times higher than that exposed at residential area with low population density (site 1) and about 2.5 times higher than the deterioration at residential areas of moderate population density (sites 2, 3). The deterioration of neat cement samples exposed to the industrial atmosphere (site 5) resulted in decrease in compressive strength from 1338 kg/cm2 for the control samples to 1158kg/cm2 (Figure 1C). In other words, the compressive strength decreased at this site by 13.45% after three years of exposure compared with strength losses of 2.86%, 6.15%, 5.85% and 9.58% at sites 1 2, 3 and 4, respectively (Figure 2). For cement block samples (Figure 1D), the compressive strength of the exposed samples at the industrial district (site 5) decreased from 261kg/cm2 to 153kg/cm2. This means that 41.38% of the strength of cement block samples was lost after three years of exposure. This loss is about 1.5 – 3 times higher than that found at the other residential areas. Compressive strength of gypsum samples show maximum decrease (from 78 kg/cm2 to 53 kg/cm2) after three years of exposure at site 5 (Figure 1E). The deterioration of gypsum samples at the industrial area is about three times higher than those at the residential areas. Figure 1F shows that the magnitude of the deterioration of LECA samples exposed at different sites in Cairo atmosphere are approximately similar to that of the sand-lime samples. Figure 2 also indicates that limestone and cement blocks demonstrate higher strength losses than the other investigated materials. However, neat cement samples show lower losses in compressive strength followed by LECA and sand-lime samples. It can be concluded that the severity of the stone samples degradation in Cairo atmosphere after three years of exposure can be arranged as follows:
Limestone ≈ cement block > gypsum > sand-lime > LECA > neat cement
Figure 3 illustrates the percentage compressive strength losses of limestone samples exposed at the chosen sites as a function of exposure times. The figure shows that the percentage loss of strength was fast during the first year and tends to be less progressive through the second and third year at all the sites of measurement. This behavior clearly indicated at site 5 (industrial area). This can be attributed to the higher concentrations of air pollutants mainly SO2 and sulfates, which can readily react with limestone forming gypsum. Due to the lack of rain in Egypt, these layers of gypsum can accumulate on the surfaces of stones forming crusts, which hinder further fast reactions. The percentage strength losses of different samples as a function of the time of exposure at the industrial area (site 5) are given in Figure 4. Figure 4 shows that, the behavior of the strength losses for the different samples can be categorized into different groups. The first group includes limestone and gypsum, where the strength loss increased at a fast rate through the first year and the rate declined through the second and third years. The second group includes cement blocks, sand-lime and neat cement, where the compressive losses rose steadily with the time of exposure. The third group includes LECA samples, where the strength losses increased steadily through the first two years and fastly through the third year.

Figure 3 Loss of compressive strength of limestone samples exposure at various sites as a function of the time of exposure.
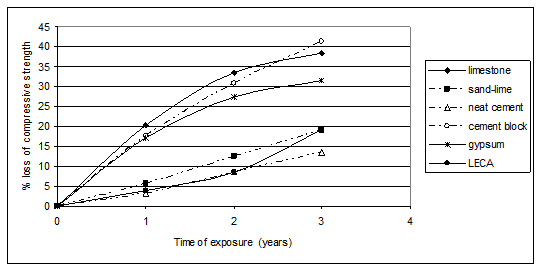
Figure 4 The strength losses of different building unit samples exposed at the industrial area (site 5) as a function of the exposure time.
SO2 doses (dose = concentration x time) were calculated for the various periods of exposure at the different sites of investigation and correlated with the compressive strength losses of the exposed limestone samples. A positive correlation coefficient (r=0.71) was obtained, this means that the stone deterioration in the atmosphere becomes far more serious if the atmosphere contains sulfur dioxide. However, an important degradation mechanism in SO2 polluted environment is therefore the conversion to gypsum followed by dissolution. This result is confirmed by.1,27–30 Furthermore, Sabbioni2 &Vallero3 reported that SO2 is for a long time considered to be the main corrosive pollutant on materials. Moreover,8–10 reported that chemical reaction on stone surface continues at all sulfur dioxide concentrations. He added that the oxidation of the SO2 aqueous phase is the key step in the formation of gypsum on the surface of the limestone. However, sulfur dioxide and moisture react with limestone (CaCO3) to form calcium sulfate (CaSO4) and gypsum (CaSO4.2H2O) (as in equations 1A and 1B):21–23
CaCO3 + H2SO4 ® CaSO4 + H2O + CO2 (Eq. 1A)
CaCO3 + SO2 + 2H2O (vapour) ® CaSO4.2H2O + CO2 (Eq. 1B)
These two sulfates are fairly soluble in water, causing deterioration in stones. The soluble calcium sulfates can penetrate into the pores of the limestone and recrystallize and expand, causing further deterioration. There is evidence also that damage occurs due to synergistic effect of sulfur dioxide and nitrogen oxides in Cairo atmosphere. Although,31–33 found that oxidation by SO2 is more significant than that by NO or NO2, in moist films on calcareous building stone, a good positive correlation (of r=0.74) found between the strength loss of limestone samples exposed in Cairo atmosphere and nitrogen dioxide dose for the various sites of measurements. Compressive strength loss of limestone samples is also correlated with the chemical constituents of deposited and suspended particulate matters (Table 2). The data of this table clearly indicate a positive correlation coefficient found with the chemical constituents of suspended and deposited particles, especially with suspended sulfates reaching 0.84. However, sulfates can attack carbonate rocks in a dual way, either by direct dissolution due to the acidic conditions or by the conversion to the more soluble sulfites and sulfates. These results confirm the major role of acid deposition in the deterioration of the exposed limestone samples. Consequently, it may be concluded that the compressive strength loss of limestone and the continuation of this loss through the periods of the study were highly influenced by the pollution load with reference to sulfate, helped with chloride and nitrate concentrations in the atmospheres of the sites under investigation. This finding is in accordance with that of.1-3,11,23,34
Chemical constituents |
Sulfates |
Chlorides |
Nitrates |
Deposited particles |
0.72 |
0.74 |
0.57 |
Suspended particulates |
0.84 |
0.63 |
0.67 |
Table 2 Correlation coefficient between compressive strength losses of limestone samples and the chemical constituents of deposited and suspended particulate matters at the investigated sites
Mineralogical Composition (X-ray diffraction)
The mineralogical compositions of the building stone films taken from the surface crust of the control samples and after exposure for three years were examined at residential and industrial sites in Cairo City by X-ray diffraction and the obtained traces illustrated in Figure 5 – Figure 10.
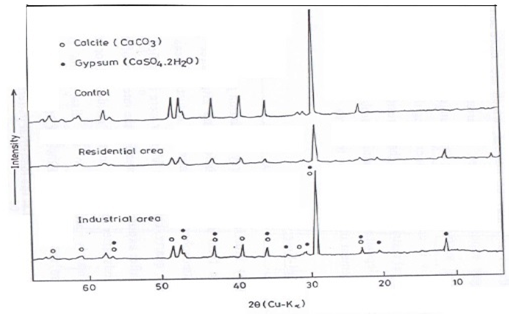
Figure 5 X-ray diffraction patterns of Limestone samples before and after exposure for three years at industrial and residential areas.

Figure 6 X-ray diffraction patterns of Sand-lime samples before and after exposure for three years at industrial and residential areas.
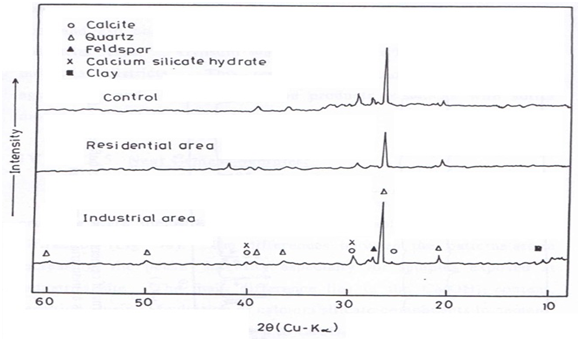
Figure 7 X-ray diffraction patterns of LECA samples before and after exposure for three years at industrial and residential areas.

Figure 8 X-ray diffraction patterns of Cement blocks samples before and after exposure for three years at industrial and residential areas.

Figure 9 X-ray diffraction patterns of Neat cement samples before and after exposure for three years at industrial and residential areas.
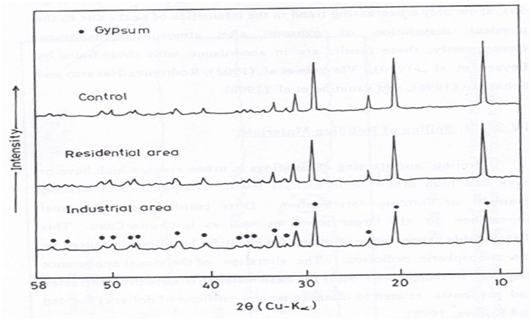
Figure 10 X-ray diffraction patterns of Industrial Gypsum samples before and after exposure for three years at industrial and residential areas.
Limestone
In the control, residential, and industrial patterns Figure 5, calcite (CaCO3) was identified as the main component. After three years of exposure, the main difference lies in the identification of gypsum, resulting from the reaction of CaCO3 with sulfur dioxide in the atmosphere that measured in high concentration at the investigated sites especially at the industrial site. Similar findings are reported by.35–39 Moreover,1–3 reported that gypsum (CaSO4.2H2O) is a secondary mineral formed from calcite in the presence of sulfate in the atmospheric environment. From Figure 5, it can be also noticed the decreasing trend of the peaks intensity after exposure owing to the conversion of calcite to calcium sulfates and may be nitrates, which are more soluble. During prolonged exposure, however, a variety of products may be deposited on the surfaces including compounds originating from the atmosphere. This is confirmed by the increase in the intensity of calcite peaks at industrial district more than at residential district due to increasing the deposited dust containing probably limestone dust especially from cement industry nearby this area.
Sand-lime
In general, quartz, calcite and feldspar were identified in sand-lime before and after exposure Figure 6. Decreasing the intensity of the peaks of these compounds after exposure to the residential or industrial atmosphere is mainly attributed to the dissociation of these compounds due to the exposure. However, the intensity of these peaks is relatively increased at the industrial pattern more than those of the residential one. This is possibly attributed to the dust deposition on the surface of the samples. The chemical effect of exposure is the dissociation of calcium silicate hydrate represented by the broad bands, found in the control pattern in the region between 8–1220, which disappeared at residential and industrial patterns. In addition, traces of gypsum were found at samples exposed at both residential and industrial districts. These results are in accordance with those of.40–43
LECA samples (Lightweight Expanded Clay Aggregates)
X-ray diffraction patterns of LECA samples (Figure 7) show that quartz, calcite and feldspar were identified in samples before and after exposure. Differences are noticed for quartz at residential site by decreasing the intensity of its peaks and approximately no change at industrial site due to further deposition. Calcite was also detected; its presence can be attributed to either of two ways: by the reaction of calcium hydroxide, Ca(OH)2, resulting from the hydration of calcium silicate in cement content, with CO2 from the atmosphere, or by atmospheric depositions containing limestone particles. In addition, deposited clays can be observed at industrial site due to the atmospheric deposition.
Cement blocks
From Figure 8, quartz and calcite only were identified before the exposure. After three years of exposure, degradation of these compounds were occurred at residential site as confirmed by decreasing the intensity of their peaks, while an increasing trend in such intensities was observed at industrial site owing to dust deposition.
Feldspar (NaAlSi3O8), which is one of dust and ash constituent, was identified in samples exposed in the industrial area. Gypsum was identified at both residential and industrial districts which resulted from the reaction of calcite aggregates and cement hydration products in blocks with sulfur dioxide in the atmosphere.
Neat cement samples
Ca(OH)2, CaCO3, and calcium silicates and their hydration products were identified in neat cement samples (Fig. 9). The differences between the patterns are in increasing the peaks` intensity especially for samples exposed at industrial site. The main difference lies in the Ca(OH)2 content, resulting during hydration of calcium silicate components in cement. Ca(OH)2 is probably transformed to CaCO3 by capturing CO2 from the atmosphere. In addition, C-S-H was observed after exposure at the industrial site. This means that hydration of calcium silicates was continued during the exposure time. Degradation of calcium silicate hydration products may be also occurred and calcium sulfate hydrate was formed.44,45 Furthermore, increasing the intensity of calcite peaks after exposure is mainly attributed to that Ca(OH)2 can absorb CO2 from atmosphere forming CaCO3, that can deposited into the surface pores of the samples in addition to the deposited particles. This may explain the lower decrease in compressive strength that measured in the present study for neat cement samples when exposed to the atmosphere.
Industrial gypsum samples
Figure 10 shows only a decreasing trend in the intensities of peaks due to the physical dissolution of gypsum after atmospheric exposure. Consequently, these results are in accordance with those found by.46–53
It can be concluded that; the deterioration in compressive strength of building materials, exposed to Cairo city through the period of study, was highly related to the atmospheric pollution load with reference to SO2, sulfate, chloride and nitrate concentrations and the severity of the reaction of pollutants on the surface of the stone samples. The higher deterioration of the building materials was recorded for samples exposed at the industrial areas compared to those exposed to the other residential districts. Limestone and cement blocks are more sensitive to air pollutants and have poor resistance to atmospheric deterioration than the other investigated stones, whereas neat cement, LECA samples, and sand-lime have much better weathering resistance. The severity of the stone samples degradation in Cairo atmosphere after three years of exposure can be discerningly arranged as follows:
Limestone ~ cement block > gypsum > sand-lime > LECA > neat cement
The sensitivity of different building materials to the atmospheric pollution can give useful information for the selection of the suitable types for construction purposes, which can resist the prevailing atmospheric conditions in Egypt. The mineralogical composition (X-ray diffraction) of the product films of the exposed building materials showed the formation of gypsum and hydrated calcium silicate components in the building materials and cement content.
None.
The authors declare that there is no conflict of interest.

©2021 Saleh, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.