eISSN: 2574-9927


Research Article Volume 1 Issue 3
Department of Chemistry, Jamia Millia Islamia, India
Correspondence: Tokeer Ahmad, Department of Chemistry, Jamia Millia Islamia, India, Tel 91-11-26981717
Received: September 01, 2017 | Published: October 13, 2017
Citation: Ahmad T, Shahazad M, Phul R. Hydrothermal synthesis, characterization and dielectric properties of zirconia nanoparticles. Material Sci & Eng Int J. 2017;1(3):100-104. DOI: 10.15406/mseij.2017.01.00017
Simple hydrothermal method has been used to synthesize zirconia (ZrO2) nanoparticles in alkaline medium. The powder X-ray diffraction technique (PXRD), electron microscopic studies (SEM and TEM) and BET surface area studies were done to characterize the as-synthesized nanoparticles. The crystalline nature and phase analysis of the ZrO2 nanoparticles was determined by X-ray diffraction studies which revealed that the as-synthesized powder is pure monoclinic zirconia (m-ZrO2). The particle size was estimated by TEM and HRTEM studies, which comes out to be about 25nm. The surface area of as-prepared ZrO2 nanoparticles was found to be 186m2/g with DA pore radius of 11.9Å by using BET surface area studies. The dielectric constant and dielectric loss of the nanoparticles were found to be 7.5 and 0.0094 for as-synthesized nanoparticles at 500kHz frequency at room temperature.
The solid materials have wide applications in many fields owing to defects in their crystal structure. The material when manipulated up to nano size then it exhibits properties very different as compared to micro size particles of the same material. Nano sized transition metal oxides have been considered gifted materials having their applications in industry, medicine and in various other fields. The metal oxide nanoparticles have attracted special attention to design nano structures for a variety of applications because of their enhanced physical and chemical properties.1 The oxide nanoparticles are used to design energy efficient lithium ion batteries,2,3 light emitting diodes,4,5 solar cells,6,7 fuel cells8,9 and transistors,10,11 hydrogen storage devices,12,13 air purification,14 water purification,15 gas sensing,16 temperature and humidity sensing studies,17 drug delivery and bio imaging studies.18,19
The nano sized ZrO2 is very interesting and valuable material for its fundamental and application based properties.ZrO2 is known to exist in three crystalline forms monoclinic, tetragonal and cubic structures. Thermodynamically most stable form of ZrO2 is monoclinic. The cubical form of ZrO2 is stable at high temperature (above 2370 °C), tetragonal form is stable between 1170- 2370 °C whereas monoclinic ZrO2 is stable below 1170 °C.20 ZrO2 is well known refractory oxide and a potential candidate as high k-gate dielectric material. To select any material as gate oxide applications, the first and very important requirement is high dielectric constant value. The material possessing too high dielectric constant value, such as for TiO2 ( 80), fringing fields from the drain through the gate dielectric are observed which may degrade the source to channel potential barrier and may lead to poor sub-threshold device functioning.13-21 Pure zirconia has been known to exhibit anionic vacancies defects predominantly. The nano sized zirconia owing to large surface area has high number of oxygen vacancies at grain surfaces.22 Therefore solid ZrO2 can conduct electricity up to some extent and it is considered as p-type semiconductor. The band gap value of ZrO2 depends upon synthesis temperature, particle size and crystalline structure. The value of band gap for tetragonal form has been reported more than that monoclinic which in turn has higher band gap value than that of cubic form.23 A number of binary oxides such as TiO2, Y2O3, ZrO2 and some perovskites materials such as SrTiO3, BaZrO3, Ba(1-x)SrxZrO3 and Ba(1-x)SrxZrO3 have been studied as dielectric oxide material for complementary metal oxide semiconductor (CMOS) devices24-27 but ZrO2 have been investigated extensively as potential candidate for dynamic random access memory devices (DRAM) owing to its moderately high dielectric constant (k = 20) and higher thermal stability.28 Numerous chemical methods have been reported to synthesize pure ZrO2, such as thermal decomposition,20 sol-gel methods,29 reverse micellar method30,31 and hydrothermal techniques.32 In this paper we have synthesized the single phase ZrO2 nanoparticles to check their applications as dielectric material as a function of frequency and temperature. The as-prepared nanoparticles were extensively characterized using PXRD, SEM, TEM and BET surface area studies.
The chemicals hydrated zirconium oxy nitrate (Alpha Aesar, 99.9%), sodium hydroxide (Merck, 99%) were used as such without further purification. The double distilled water was used throughout the experiment.
The synthesis of ZrO2 nanoparticles have been processed using typical hydrothermal method. 0.1M ZrO(NO3)2.xH2O and 0.2M NaOH were prepared in double distilled water. Equal volumes (25ml of each solutions) were mixed in order to form a 1: 2 molar ratio solution of ZrO(NO3)2.xH2O and NaOH. The solution obtained were transferred in to the hydrothermal flask and placed in oven for heating at 150 °C for 4hours. To collect the precipitates, the solution was centrifuged for 5 minutes at 8000rpm and washed with acetone several times. The precipitates were then dried in vacuum oven at 110 °C for 90 minutes and grinded to obtain fine powder of ZrO2 nanoparticles.
The crystalline structure of as-synthesized nanoparticles was determined with the help of Rigaku ultima IV X-ray Diffractometer with Cu target and ( ) = 1.5416Å. Powder X-ray diffraction patterns were recorded in 10-80° 2q scanning range with scanning rate of 0.05° per second. The morphology of the nanoparticles was determined by scanning electron microscope FEI SEM (Model: Nova Nano SEM 450, Hillsboro, OR). The powder obtained after grinding the sintered pellet was used to get SEM images at various magnifications. The average particle size of as-synthesized ZrO2 nanoparticles was determined by transmission electron microscope FEI Technai G2 20 TEM by using accelerating voltage of 200kV. TEM specimen was prepared using fine powder form of the sample. The nanopowder was dispersed in absolute ethanol and sonicated by an ultrasonic bath for 30 minutes. The dispersed sample was placed on a carbon coated copper grid with the help of micropipette. The grid was dried at 100 °C for 1 hour. BET surface area analyzer (Model: Nova 2000e, Quantachrome Instruments Limited, Boynton Beach, FL) was used for surface area studies of the sample. The specific surface area of as-synthesized ZrO2 nanoparticles was determined using “Multipoint BET Method” at 77K. Before analysis for specific surface area, 0.05g of the sample was degassed in vacuum degassing mode for 3 hours at 250 °C to take out absorbed water vapours and gaseous contaminations. A known amount of adsorbing N2 gas got admitted into the sample cell having powder adsorbent. In the sample cell, the pressure changes to get equilibrated during the adsorption. The absorption plot was used to compute the specific surface area of the sample by using BET principle. Dielectric measurements of as-synthesized nanoparticles were performed with the help of high frequency LCR meter (Model: 6505P; Make: Wayne Kerr, UK). Before performing the dielectric measurements, the diameter and thickness of the pellets were measured and area of pellets was calculated. The capacitance and dielectric loss were recorded by using sintered pellet of the sample and dielectric constant value was find out from the measured capacitance of the sample.
The phase purity and the crystal structure of as-prepared nanoparticles were identified by Powder X-ray diffractometer. Figure 1 represents the diffraction pattern for zirconia nanoparticles which is indexed as monoclinic ZrO2 (m-ZrO2) and are in good agreement with the standard JCPDS no. 83-0936. The diffraction pattern shows sharp and well defined peaks which indicate the highly crystalline nature as well as purity of the sample. Thus on the basis of X-ray diffraction studies, it has to be concluded that pure monophasic ZrO2 powder was prepared by using the prescribed method as discussed in experimental section.
The surface morphology of the as-acquired zirconia nanoparticles was investigated by scanning electron microscopy as shown in Figure 2. SEM image clearly indicates that the zirconia nanoparticles has smooth surface with small particle size. The agglomeration of nanoparticles is also visible in the SEM images.
The shape and size of the as-prepared nanoparticles have been estimated from transmission electron microscopic (TEM) studies. The TEM micrographs of zirconia nanoparticles are shown in Figure 3a. The micrograph shows that the particles are uniform in size and the small spheres tend to form cubic shape. The average size estimated from these micrographs was found to be 25nm. The transmission electron micrograph is in good agreement with the SEM image. Figure 3b represent HRTEM image of as-synthesized monoclinic ZrO2 nanoparticles. The HRTEM micrograph visualizes well-resolved lattice fringes at a distance of 2.84 0.04Å which can be accredited to the inter-planar spacing corresponding to (111) plane.33 The parallel fringes were found to be equidistant authenticate single phase nanocrystalline grains of monoclinic ZrO2. The selected-area electron diffraction (SAED) pattern of zirconia nanoparticles is shown in figure 3c which demonstrates the different lattice planes of m-ZrO2 (ˉ111), (111), (122) and (131) in the SAED pattern which are in good agreement with the XRD pattern.
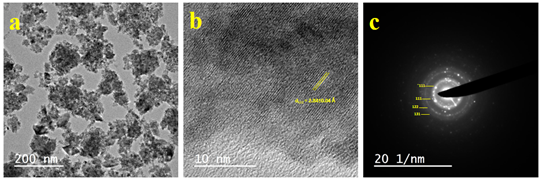
Figure 3 (a) Transmission electron micrograph, (b) HRTEM image and (c) SAED pattern of ZrO2 nanoparticles.
The specific surface area of the as-acquired nanoparticles was determined by the Brunauer-Emmett-Teller (BET) gas adsorption method34 and the pore radius and pore volume was calculated using Barrett-Joyner-Halenda (BJH) method.35 Figure 4 shows the BET plots of zirconia nanoparticles. The specific surface area calculated using the multipoint BET equation was found to be 186m2 g-1. Pore size distribution plot of as-synthesized nanoparticles is shown in Figure 5 & 6. The pore radius of these nanoparticles was calculated using BJH method as well as DA method and presented in Table 1 with the corresponding pore volumes.
Nanoparticles |
BET Surface Area [m2g-1] |
BJH Pore Radius [Å] |
DA Pore Radius [Å] |
Pore Volume cm3g-1 |
|
ZrO2 |
186 |
30.9 |
11.9 |
0.247 |
Table 1 BET surface area, BJH and DA pore radius parameters of as-synthesized nanoparticles.
The dielectric properties of as synthesized nanoparticles were studied as a function of frequency after sintering the pellets at 1000 °C for 10 hours. The dielectric constant is estimated by using the formula.36
Where C is capacitance of pellet, d the thickness of pellet, A the cross-sectional area of the flat surface of the pellet and ε0 the permittivity for free space.
The dielectric characteristics including the dielectric constant and loss factor were determined with variation of frequency in a range from 20KHz to 1MHz at room temperature as shown in Figure 7. Figure 7 shows that the dielectric constant decreases with increase in frequency. On account of space polarization effect, the zirconia nanoparticle shows high dielectric constant at lower frequency region. However, at higher frequency region the polarization effect reduces which raises the decrease in dielectric constant.37 The room temperature dielectric constant and dielectric loss values were found to be 7.5 and 0.0094 for the as synthesized nanoparticles at 500kHz.
Zirconia nanoparticles were synthesized by the simple hydrothermal method in alkaline medium at 150 °C. X-ray diffraction, HRTEM and SAED analysis confirms that the synthesized nanoparticles are pure monoclinic phase of ZrO2. The diameters/particle size of the m-ZrO2 nanoparticles fell into range of 25nm with the specific surface area of 186m2g-1. The SEM image is in accordance with the TEM micrograph. The dielectric properties of m-ZrO2 nanoparticles make them a suitable material for the storage devices and electronic devices. These nanoparticles can also be used for photonic applications, gas sensors and solid oxide fuel cells.
TA thanks to CSIR, Govt. of India for financial support of the research project (No. 01(2897)/17/EMR-II). The authors thank CIF, Jamia Millia Islamia for X-Ray diffraction studies and JNCASR, Bangalore for electron microscopic studies. MS and RP especially thanks to UGC, New Delhi for Research Fellowship.
The author declares no conflict of interest.

©2017 Ahmad, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.