eISSN: 2574-9927


Research Article Volume 3 Issue 1
1Department of Physics, Banaras Hindu University, India
1Departamento de Química, Universidad Nacional del Sur, INQUISUR-CONICET, Argentina
Correspondence: Grupo de Fisicoquimica de conductores iónicos de estado solido, GFCIES, Departamento de Quimica, Universidad Nacional del Sur, INQUISURCONICET, Av. Alem 1253, CP 8000 - Bahía Blanca, Argentina
Received: December 22, 2018 | Published: January 29, 2019
Citation: Prátula PE, Pistonesi CA, Anton MA, et al. Electrical and magnetic response of a phosphate glass - NiFe2 O4 composite. A novel magnetic sensor design. Material Sci & Eng. 2019;3(1):13-19. DOI: 10.15406/mseij.2019.03.00082
NiFe2O4 spinel – phosphate (Bi-Ba-Li) glass composite was synthesized by solid-state reaction. New crystalline magnetic phases was developed inside the glassy matrix through a controlled heat treatment. New NF-LBPB4 magnetic nanocomposite material was study. Complex impedance analysis has shown that mobile ions inside the matrix induce the development of complementary crystalline phases resulting in a composite material with excellent magnetic response. An innovative homemade device was designed to test the nanocomposite magnetic response.
Nowadays, magnetic nanoparticles materials increase their interest because they lead to new technological applications. A step forward is magnetic-glassy nanocomposite materials because they give the opportunity to obtain devices with very different shapes retaining their electrical and magnetic behaviour. Additionally, glassy matrix acts as a protection of the nanocrystalline magnetic material.1–4 Glass-ceramics combined with multi ferroic phases have many device applications as control the magnetic state via local electric fields, the combination of ferroelectric and ferromagnetic phases allows store information on the basis of their polarization5 encoded in the direction of the electric polarization and magnetization, yielding spintronics and non-volatile data storage, multistate permanent memory device.6,7 Some works have reported the development of such materials: Bi2O3–Fe2O3–BaO–B2O3 in which BiFeO3 phase was crystallizable given a glass-ceramics;8 nanocomposites containing nanocrystals of Te2NiMnO6 synthesized by heat treatment9 etc. The synthesis of nanoparticles is a complex process, and there are many different techniques for producing them.10 Liquid phase synthesis remains one of the most common methods by the co-precipitation method. Nanoparticles can be oxide nanoparticles produced directly and by a precursor that is then subjected to an additional post-processing.11 In this work, we have synthesized NiFe2O4 by the solid-state reaction method followed by a combination with a 42Li2O 18BaO (36P2O5 4Bi2O3) glass that allows us grows new crystalline magnetic phases inside the glassy matrix through a controlled heat treatment resulting in a magnetic nanocomposite material. We have study its structural features and its magnetic its magnetic and electrical behavior.
NiFe2O4 [NF] synthesis
In this work NF (NiFe2O4) pure powder was prepared by solid-state reaction method. NiCO3 (II) (Riedel-de Haen AG. Seelze-Hannover) and Fe2O3 (Sigma-Aldrich ≥99%) powder were use to synthesize NF. Starting from the nominal composition every component was weighted and mixed in an agate mortar. The powders were well mixed and placed in a platinum crucible and decarboxylated at 8000C for 2h in an air atmosphere and followed by 2h at 1100oC. The obtained powder was pressed in pellets of 10 mm diameter and 2mm thickness at 10.2 metric ton/cm2 and sintered at 700oC for 2h. Finally, the pellets were grind again.12–15 The crystal structure was characterized by X-ray diffraction (Philips X Pert Pro) in continuous scan mode with a copper anode (45K-30mA, Cu Kα radiation: λ=1.54Å ) at room temperature in the 2θ range: 10°-90°.
Phosphate glasses synthesis
Ternary glasses of composition: 19BaO (74P2O5 7Bi2O3) and 42Li2O 18BaO (36P2O5 4Bi2O3) (mol%) was prepared by a standard melt quenching technique. From now [LBPB] and [LBPB4].16
Composites NF-LBPB synthesis
The prepared glasses were ball-milled and the resulted powder was sieved with a 150μm mesh sieve to obtain the small fraction of particles. The glassy powder was mixed with NF powder in a ratio of: 89%wt/11%wt. The mix was heated at 700oC for 30min.
Composite NF-LBPB4 synthesis
Powdered [LBPB4] glass was mixed with a [NF] in a ratio of 89%wt to 11%wt respectively. Both powders were mixed in an agate mortar a new glassy material was prepared by the standard melt quenching technique. First, it was heated for 1 h a 1353 K, shaken frequently to ensure homogenization. The melt was poured on an aluminum plate at 0oC and the obtained solid drops were held for two hours a 473K to remove the mechanical stress and 3hrs at 703K to allow the grows of [NF] crystallites inside the glassy matrix.17,18
The glassy nature of the resulting solids by quenching technique was tested using X-ray diffraction (XRD) analysis and differential temperature analysis (DTA). DTA curves were recorded using a Rigaku Thermoflex TG 8110 associated with Thermal Analysis Station TAS 100, the Tg (glass transition temperature) of each sample was determined with a heating rate of 10K min−1 in the range 25ºC to 400ºC using 20–30 mg of glass samples finely grounded in an agate mortar.19 Figure 1 shows the X-ray diffraction patterns that confirms the single phase material. All the diffraction peaks can be indexed to the cubic spinel structure of JCPDS card no. 74-2081 with the presence of (220), (311), (400), (422), (511), (440), and (622) planes, with small proportion of impurities. The lattice parameter was in good agreement with standard value. Figure 2 & Figure 3 show the DTA scan and the corresponding Tg for each glassy sample. From the results in these figures we learn that the Tg value decreases 10% when lithium oxide is incorporated in the phosphate glassy matrix. Also, Figure 3 shows two extra peaks after its Tg which belong to the devitrification phenomenon.

Figure 1 [NF] X-ray diffraction pattern (pink); NF/LBPB composite (blue) X-ray diffraction pattern; [LBPB4] (red) and [LBPB] (black) X-ray diffraction pattern.
Electrical properties
Every glassy sample was polished with a sand paper to obtain disks with two parallel mirror surfaces with thickness ranging between 0.5 to 0.7mm and coated with a thin layer of silver paint to having a proper electrical contact. Impedance measurements were carried (Agilent LCR meter 4284A; 20Hz–1MHz; AC voltage amplitude signal of 0.80V) in a temperature range between room temperature and (Tg−15)°C. Electrical measurements was done registering the magnitude of the sample impedance |Z| and the phase angle f, using the impedance meter. Show the complex permittivity e’(w) and the dielectric loss (tan δ) measured as a function of the temperature for the pristine composite NF-LBPB4 and NF/LBPB4 treated 1 h to 703K. The values of the complex permittivity are calculated using the following equations:
(1)
(2)
In these expressions ε’ is the real part of the complex permittivity characterizes the energy stored in the material and, ε’’ is the imaginary part and represents the energy losses by the material;
ε0 is the free space permittivity (8.854*10-12 F. m-1); (L/A) the geometric factor; f, the frequency.
The dielectric loss is obtained from the relation: (3)
Figure 4 shows that the ε’ is lower when the material is heat treated and also the dielectric loss which decrease more confirming lower energy losses than in the pristine sample. The Maxwell - Wagner model for the heterogeneous structure explain σ the ε’ dispersion phenomenon observed at low frequency. We assume that the grain boundary present in the composite are smoothed after the heat treatment due to a cation interchange between the combined materials. Additionally, Figure 5A & Figure 5B show that after the heat treatment crystallites are larger enough to be revealed by a second resistance response clearly observed in Figure 5B. However, as we mentioned above the interface between both materials is less bloquing as the conductivity spectra showed in Figure 6A & Figure 6B while the σdc is low after the heating confirming that mobile ions in the glassy matrix are trapped around the crystalline phase given new crystalline structures as X-ray diffraction pattern shows in Figure 1.

Figure 4 Real part of the complex permittivity of: A)NF-LBPB4 pristine composite and B) NF-LBPB4 treated 1 h at 703K.
Magnetic properties
Figure 7 shows the magnetization loops (T-H) measured at room temperature.20 It exhibits typical ferromagnetic behavior and it reaches a saturation magnetization values 3 Ms (pristine composite; glass ceramic) ≈ Ms (heat treated composite, after 1 or 2 hours). The composite prepared as a glass-ceramic has the same response as the pristine composite. In order to obtain such response we have design a homemade magnetic analyzer. A sensor with a fine adjustment screw type system was built like describe the following Figure 8(A-D), using the same circuit, the measurement is made with air core and winding of 100/100 turns.21,22 The magnetization curve of each material is determined based on which the flow is determined by the supply voltage. The circuit used is shown in Figure 8:

Figure 7 Magnetic hysteresis loops at room temperature of NF-LBPB4 composites pristine and heat treated at different times.

Figure 8 A) Sensor design; B) Sensor built; C) sample location; D) oscilloscope ScopeMeter Fluke199) 200 MHz 2.5 GS / s, double input.
(4)
(5)
The current is measured on the primary winding and the voltage on the secondary winding, thus achieving that both values are not affected by the insertion of the instruments. The current determines the magneto motive force and the voltage the conversion flow. A device was designed to check the operational material behavior using 0.27mm thick oriented grain iron as reference. The system has the following components:
The device has a 24 volt direct current motor, rack and chain system that convert the rotary movement into linear. The system has a permanent magnet and limits of career and position sensors for the movement and measurement.23,24 Also, the system has a second element where the samples are placed and where is the voltage induced. The voltage generated by the samples is compared to the passage of the permanent magnets. We use first silicon steel whose characteristics are well known and next our glassy composite materials. It is important to emphasize that both shape and dimensions of samples are thoroughly determined. The generated voltage on the driver depends on the magnetic field intensity due to the speed of the magnets and the magnetic circuit that surrounds the driver. As an example, Figure 10 shows the result of one measure.
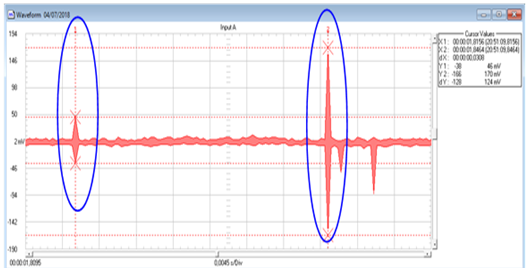
Figure 10 Comparative voltage measured of two series conductors: silicon steel—glassy composite material (NF-LBPB4).
First signal of 7.20 10-3 s – Intensity: from 2mV to 50mV
Second signal of 8.40 10-3 s – Intensity: from 2mV a 194mV
We took 9727 measurements during 3843 seconds in order to obtain an average response.
The growth of the crystalline phase inside the glassy matrix improves the magnetic response of the composite material. The resulting material’s Tg is high enough to permit the proposed technological use. From the electrical results we show that a smooth inter phase between the magnetic crystallites and the glassy matrix is obtain after an appropriated heat treatment assisted by the cation interchange. The crystallites obtained are larger enough to be observed and the mobile ions in the glassy matrix trapped around the crystalline phase give a new crystalline structures. As a result, the composite material developed has the advantages of the glassy materials with the magnetic properties necessary to be applied in green electrical generation for example (as part of a stator). The innovative homemade device designed allows the magnetic characterization of our composite material. Considering that it was built with standard laboratory equipment, it is an excellent opportunity to test the material performance in comparison to very well known magnetic materials as for example silicon steel that we used.
In this work we synthesized the NiFe2O4 spinel by solid-state reaction and the phosphate (Bi-Ba-Li) glass by melt quenching technique. Applying a mechanical mix and several heat treatments an innovative nanocomposite was developed. Additionally, the heat treatments led to growth new crystalline magnetic phases inside the glassy matrix. The NF-LBPB4 magnetic nanocomposite material was thoroughly study. Complex impedance analysis allows to evidence that are the cations inside the matrix which induce the development of complementary crystalline phases. The composite material obtained has an excellent magnetic response. In order to evaluate the composite magnetic behavior an innovative homemade device was designed. We present technical details to build it.
Financial support by CONICET (PIP 11220120100010CO), ANPCyT (PICT 2016-0101), UNS. M.MdeL.S. CPI English assistance. P.E.dP. is CONICET fellowship and M.A.F is CONICET Research Fellows, Argentina.
Author declares that there is no conflict of interest.

©2019 Prátula, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.