eISSN: 2574-9927


Research Article Volume 1 Issue 3
1Department of Metallurgical and Materials Engineering, Federal University of Technology, Nigeria
2Chemical Engineering Department, University of Johannesburg, South Africa
Correspondence: Olaniran O, Department of Metallurgical and Materials Engineering, Federal University of Technology, Nigeria, Tel +2348035001939
Received: August 24, 2017 | Published: September 28, 2017
Citation: Olaniran O, Folorunso DO, Oke SR, et al. Effect of zirconia dispersion on mechanical and corrosion properties of ductile iron. Material Sci & Eng Int J. 2017;1(3):95-98. DOI: 10.15406/mseij.2017.01.00016
The increasing applications of ductile iron in most of the major industries, especially in marine, food processing, chemical and electrochemical, agriculture and petroleum industries have made them versatile construction materials. Their good mechanical and chemical properties, chemical composition, weld ability, ease of casting, provide the best-round performance at relatively low cost. This work focuses on the production, mechanical and corrosion studies on ductile iron composite. Ductile Iron composite was produced by sand casting process with varied percentage composition of zirconia. The influence of the zirconia reinforcement on the mechanical; hardness and tensile, properties were investigated. Similarly, the effect of the zirconia on the corrosion property of the composite in 3M NaCl solution at room temperature was studied. From the results, it was observed that the tensile strength and yield strength decreases with increasing percentage composition of the zirconia reinforcement. However, dispersion of the zirconia reinforcement within the matrix of ductile iron considerably improved the corrosion resistance of the composite.
Keywords: ductile iron, composite, reinforcement, corrosion, strength
The wide application of ferrous materials is attributed to its amenability to alloying and heat treatment which makes it possible to modify its properties to suit specific service requirements. Among such applications is the development and modification of ductile iron with zirconia to enhance its strength and corrosion resistance, and this could be used to a large extent in the production of agricultural implements and some automobile applications. Industries have discovered various materials and process combinations that exhibit surprisingly good strength, wear and corrosion resistance materials for longer life. Ductile iron has properties that when worked upon, could make it to be a possible replacement for forged steels in many applications,1 Janerkaet al.2
The industrial potency of any nation is predicated to a large extent on its steel technology of which ductile is a good alternative; it is thus a source of concerned that in most third world countries, the steel technology is still very low. Therefore, agricultural development depends partly on agricultural implements which are made of ductile iron. For such applications, high wear resistance, strength, toughness and corrosion resistance are some of the vital engineering properties that are needed for excellent service performance.3 The usual ductile iron has been observed to have limited value on those properties, hence the need for modification.
Corrosion of metals is a destructive process regarding to basic modern constructional materials with a great importance for nowadays industry and in many cases represents an enormous economic loss Olaniran et al.4 The overall cost and environmental implications of corrosion problems has become a major challenge to engineers in the fight against corrosion and the quest to reduce economic loss. Corrosion is one of the leading causes of structural damage and failure to engineering materials. Of particular importance is pitting and inter-granular corrosion, which can develop into fatigue cracks, stress corrosion cracks. Therefore, it is not a surprise that the research and several approaches on the corrosion and corrosion protection of metallic materials such as cathodic protection, coatings, and corrosion inhibitors have been developed on a large scale in different directions and a wide range of engineering decisions to mitigate corrosion Potgieter et al.5 However, due to the fact that these approaches are external, attention should also focus on the selection of proper corrosion resistance alloys for specific industrial applications. The improvement of corrosion behavior of metals and alloys still stays as one of the fundamental engineering problems in the area of material selections and applications, and it is one of the important parts of modern surface engineering. Special attention is focused on the corrosion behavior of ductile iron as vastly used engineering materials, as it has limitation to corrosion resistance. The resistance of ductile iron depends among many other factors, on its passive nature, alloy chemistry and the specific environment to which it is exposed. The inert nature of the passive film on the surface is dependent on its stability in the medium of exposure Behpour et al.6 A good strategy in improving corrosion resistance is by carefully monitoring the alloying contents in metal. Zirconia is known for their resistance to oxidation and corrosion due to their high activation energy Pawawoi et al.7 Thus, studies have shown that the corrosion resistance of cast iron can be improved by alloying them with small additions of PGM Sherif et al.8 This approach in improving the corrosion resistance of a base alloy by adding a little quantities of Platinum Group Metals 9 (PGM) is known as cathodic modification potgieter et al.5 Studies have also shown that ZrO2 films prepared by sol-gel methods with densification at high temperature (800 °C for 2hrs) act as efficient corrosion protectors of 316L SS in NaCl and H2SO4 solutions. Furthermore, only a few investigations had been carried out on the corrosion behavior of ductile cast iron in salt solutions. This study therefore investigates the effect of addition of small amounts of zirconia and its possible synergistic effect on the mechanical and corrosion properties of cast ductile iron in sodium chloride environment.
Materials
The materials used for this work are white silica sand, bentonite, water, cylindrical pattern, scraps engine block, zirconia powder of 50nm particle size and Ferrosilicon.
Method
The mould was prepared from white silica sand (80%) mixed with (10%) bentonite as binder and (10%) water to activate the clay into paste. The prepared mould was dried to remove moisture and pattern removed to create cavities for casting. Scraps of cast iron engine block were weighed for melting using a HANA weighing balance. Charge calculations were utilized to determine the various quantities of materials to be charged into a gas-fired pit furnace. Ferrosilicon was added and the furnace heated to a temperature of 1500°C ± 30 above the melting temperature. Preheated zirconia powder was added at this temperature while stirring of the slurry was performed manually for 10 seconds to help achieve homogeneous dispersion of zirconia powder in the molten metal. It was further reheated to 1600 °C. The composition was then de-slag and tapped into the ladle before pouring into the prepared mould. Unreinforced ductile iron was also prepared for control experiment and labeled as sample A while variations of 0.2 vol%, 0.5 vol%, 1 vol% and 2 vol% zirconia reinforcement were labeled as B, C, D and E respectively. The cast was left in the mould for five hours for complete solidification before finally removed.
Mechanical tests
Tensile test was carried out on both control and the zirconia dispersion strengthened steel using ASTM – E8/E8M (2016) standard; the samples for the test were machined to round specimen configurations with 5mm diameter and 30mm gauge length. The tensile test was carried out at room temperature using an Instron universal testing machine operated at a strain rate of 10-3/s until fracture. Hardness tests were also carried out using LECO Rockwel Micro hardness Tester model 4150AK at a dwelling time of 10 seconds by cutting the specimen to gauge length of 30 x 19.7mm diameter and properly grinded and polished. Five different tests were performed for each test condition to guarantee reliability of data generated. Average values were calculated and recorded.
Electrochemical analysis
Samples were prepared for electrochemical analysis using ASTM-G5 standard (2011). The surfaces of the samples were ground, polished and washed with distilled water, degreased in acetone and finally dried in air. The electrochemical behavior of these samples were studied using Princeton Autolab Potentiostat (Model PGSTAT 204N computer controlled) and the data were analyzed by the general purpose electrochemical Nova software (GPES) version 4.9. The set up was done using 3.0% NaCl solution at room temperature. The polarization10 curves were measured at a scan rate of 0.2mV/s. The electrolyte was replaced after each scan.
The variation of ultimate tensile and yield strength are presented in Figure 1 which shows clearly that the ultimate tensile strength and the yield strength of the composite decreases with increase in volume percent (vol %) of zirconia addition as a result of brittle nature of zirconia. Proper stirring and absence of temperature gradient helps in achieving a refine and homogeneous structure by redistributing the particle within the metal matrix. It also helps in considerable elimination of particle clusters and segregation. The composites decrease in strength as the volume of zirconia increases but little bit increases in brittleness and less tough under tensile loading. Cold roll and solution heat treatment process can help in restoring the ductility of the material. It could also enhance the strain hardening capacity of the composites Figure 2. This could also reduce the gas porosity in the material (Alaneme, 2010).
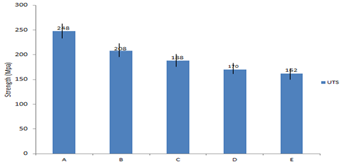
Figure 1 Variation of ultimate tensile strength and yield strength for composite produced with varying composition of zirconia.
From the chat in Figure 3, it was observed that the hardness values increases with increase in the volume of ZrO2. The composite containing 2% ZrO2 have the highest hardness value. This is due to higher concentration of zirconia particles within the matrix of the metal. As the quantity of zirconia addition increases, there is an appreciable progressive increase in the hardness values, the reason for this is that zirconia particles is a hard ceramic material and evenly distributed in the same volume of metal matrix. Hence in combination with the presence of carbide11 in the iron, there will be a remarkable increase in the hardness, possible thermal stability and better wear resistance since zirconia is a form of ceramic material that is hard and strong Glage et al.,12 Olaniran et al.13
The potentio-dynamic polarization for the ductile iron and its alloy were investigated in 3% NaCl (Figure 4) in order to study the effect of ZrO2 on the corrosion behaviour of the ductile composite. Table 1 shows the Ecorr, Icorr, tafel slopes and corrosion rates obtained. The results show that the samples alloyed with 2% ZrO2 exhibits the highest corrosion resistance in NaCl amongst the composites. The corrosion potential of the samples alloyed with 2% ZrO2 moved to a less negative potential (from 0.66229V to 0.59948V). This could be as a result of the structure-property characteristics of zirconia (chemical innertness) which invariably was high enough to passivate the composite as a result of the quantity. Varying degrees of reinforcement of the mild steel with ceramic oxide show varying degree of corrosion depending on the quantity and the ability of the reinforcement to passivate the composite. The corrosion current densities of the composites were found to increase with increasing amount of ZrO2.
|
Ecorr |
Icorr |
ba |
bc |
Corrosion Rate (mm/yr) |
Polarization Resistance ( ) |
Control (A) |
-0.66160 |
6.33E-06 |
0.61183 |
0.138602 |
0.77178 |
1560.2 |
0.2% ZrO2 (B) |
-0.64925 |
6.64E-05 |
0.60161 |
0.12811 |
0.73605 |
1732.2 |
0.5% ZrO2 (C) |
-0.63909 |
2.55E-05 |
0.42503 |
0.16753 |
0.29622 |
1995.5 |
1% ZrO2 (D) |
-0.64530 |
2.52E-05 |
0.43731 |
0.13249 |
0.29282 |
2070.9 |
2% ZrO2 (E) |
-0.59619 |
8.51E-05 |
0.10221 |
0.04359 |
0.09883 |
2569.6 |
Table 1 Corrosion Rate calculated from the potentiodynamic scan of samples A-E.
Consequently, the corrosion rate of the composites decreased with increasing amount of ZrO2 addition. Hence the results indicated that the addition of calculated amount of ZrO2 into the ductile iron can result in good corrosion resistance of the composite compared to their wrought counterpart as this is also evident in Figure 3 by shifting the corrosion potentials to the positive value. When a metal corrodes, the rate at which it corrodes is generally controlled by the cathodic reaction and their susceptibility to chloride ions.
Zirconia addition was found to reduce both the ultimate tensile strength and yield strength of the developed composites because the base metal and the dispersant are both brittle. However, the hardness of the composite was increased. The corrosion of ductile iron composites having varying composition of ZrO2 after exposure to 3% NaCl solution was also studied using convectional electrochemical method. Polarization scan shows large positive shifts in the corrosion potential of the samples with the increasing presence of ZrO2. The addition of calculated amount of zirconia increases the resistance of the composite against corrosion. Hence, cathodic modification can increase the corrosion resistance of ductile iron in 3% NaCl solution by increasing the potential to a value that is in the passive potential range. The results show that ZrO2 addition to ductile iron is a promising method for improving its corrosion resistance in chloride environment.
None.
The author declares no conflict of interest.

©2017 Olaniran, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.