eISSN: 2574-9927


Research Article Volume 2 Issue 6
Departamento de Química, Universidad Nacional del Sur, Argentina
Correspondence: Frechero MA, Departamento de QuímicaUniversidad Nacional del Sur, INQUISUR- CONICET, Av.Alem 1253, Bahía Blanca, Buenos Aires, Argentina
Received: July 31, 2018 | Published: November 21, 2018
Citation: Cardillo E, Molina MC, Sola ME, et al. Effect of small mobile cations on molybdenum-borate glasses. Material Sci & Eng. 2018;2(6):199–204. DOI: 10.15406/mseij.2018.02.00057
Lithium borate glasses modified with molybdenum oxide according to the formula: xMoO3 (1-x)[0.25 Li2O 0.75 B2O3] prepared by the melt quenching technique were studied. Density, differential thermal analysis, FTIR and impedance spectroscopic were used to analyze these systems. It was confirmed that the glassy matrix of these systems was based on the MoO6 octahedral units and on BO3 and BO4 units. The results explained how the stability of a borate matrix that hosted molybdenum ions was affected by the presence of small mobile ions (lithium ions in the present case). It was found that concentration of molybdenum oxide was restricted by strong interactions between the charged molybdenum structures and the mobile ions due to the presence of order forces that promote the formation of crystalline structures.
Keywords: molybdenum, oxide glasses, electrical properties, borate glass stability
In the field of glass technological applications it is fundamentally important to understand the influence of the structure on the glass properties. It seems curious to speak about the structure of glasses, but it is acknowledged that the three-dimensional order of components is not random in the case of oxide glasses. Modified glasses with transition metal ions are interesting in the spectroscopic field because their properties make them suitable for optic fibers, Fresnel lenses, etc. Binary glass systems based on Na2B4O7 modified with different transition metal oxides have already been thoroughly studied elsewhere.1–3 Nowadays, these glasses are of interest for laser and infrared detection techniques.4 B2O3 is a basic glass former because of its higher bond strength, lower cation size and small heat of fusion.5 Also, a large set of crystalline borates as: BaB2O46, BiB3O6,7 SrB4O7,8 CsLiB6O10,9 LiNaB4O710 have been extensively studied because of their particular physical properties in non-linear optical devices. Li2O-B2O3 system also yields Li2B4O7 compound which is technologically important due to its surface acoustic wave (SAW) and its piezoelectric and pyroelectric properties.11,12 Although the optical properties of LiB B2O5 have been studied in detail,13,14 the literature on the electric properties is limited.15 Taking into account that molten boron oxide does not crystallize even when it is cooled at a slow rate, it can be considered as the best glass maker because it is an excellent network former in which its coordination can be either threefold and/or fourfold. However, the glassy network features given by this oxide depends on its composition (i.e. on the glass former oxides and/or modifier oxides incorporated) and the melting conditions applied. Since B in [BO3/2] is electron deficient (in a non-covalently bonded [BO3/2] unit) it can accept two more electrons. This happens when an oxide ion is available in the glass composition for such additional bonding. Consequently, [BO4/2]- units are readily formed in tetrahedral borate glass structures.16
The molybdenum ions incorporated in glasses are useful because of their catalytic properties. Molybdates crystallization during the borosilicate melts cooling containing more than 4wt% MoO3 has been formerly researched. Others glassy systems like SiO2–Al2O3–B2O3–Na2O–CaO has a limited solubility of MoO3 au to 5.6 wt%. When the molybdate amount increases a crystallization process is observed during glass quenching. Since molybdenum is a fission product formed during the production of electricity in a nuclear reactor, a safer reprocessing nuclear spent fuel is mixed with other fission products and actinides in high-level nuclear waste solutions (HLW) before being confined in highly durable borosilicate glassy matrices. Bardez et al. show that the rare earth-rich borosilicate glass composition is able to incorporate 2.3wt% MoO3 without molybdates crystallization during cooling.17 Understanding how molybdenum ions behave in a glass is fundamental to increase their nuclear waste loading. Literature displays many studies on the structure, properties and mechanisms of conduction of glasses formed/modified by MoO3.17,18 As Mo−O bond in MoO3 is covalent, the molybdenum ion usually appears at least in two oxidation states: Mo+5 and Mo+6 in a glass network.19,20 ESR studies on glasses with molybdenum ions have shown the presence of octahedral coordinated ions along with distorted octahedrons approaching tetragons.21,22 These ions can work as network formers and also as network modifiers depending on the chemical composition of the glassy matrix and the oxide which acts as network host. In a B2O3 based glass the Mo6+ ions are likely to be in the glass network as MoO42- tetrahedral units alternating with BO4 tetrahedral units. Therefore, in order to find the best stable glassy matrix containing large quantities of molybdenum, the influence of MoO3 concentrations on the framework and its relationship with the lithium mobile ion content in a borate glass is described in some detail in the present work.
Materials were prepared by melting a mixture of weighed amounts of reagents-grade chemicals, namely MoO3, B2O3 and Li2CO3 in a platinum crucible at a temperature of 950ºC for 2 hours. To ensure homogenization and the complete gas liberation of the carbonate decomposition, the mixture was stirred several times. Molten glass was poured in drops onto a preheated aluminum plate at 200ºC. The nominal composition of the prepared glasses is given in Table 1. The electrical conductivity of these materials was determined by a.c. impedance spectroscopy with an Agilent 4284A LCR meter in the frequency range from 20Hz to 1MHz in a temperature domain from 60ºC to (Tg – 15) ºC. A glassy disk with parallel faces was obtained by polishing a drop. After that, each face of the cylinder was coated with a fine layer of silver paint in order to have proper electrical contact. Differential Thermal Analysis (DTA) measurements were carried out applying a heating rate of 10 K.min-1, in a temperature range of 298 K to 1273 K on a Rigaku Thermoflex TG 8110 associated with Thermal Analysis Station TAS 100. X-ray powder (XRD) was performed with a PW1710 BASED instrument in continuous scan mode with a copper anode and 45 KV– 30 mA for the tension and electrical current generator respectively. Samples were exposed to the Cu Kα radiation (λ = 1.54 Å) at room temperature in the 2θ range: 10º- 60º. For FTIR spectra, each sample was previously ground in an agate mortar to obtain a very fine powder. A semi-quantitative dispersion of each powdered sample in Nujol was registered on a Nicolet Nexus FTIR instrument, in the 2000–400 cm-1 range, at room temperature, using KBr windows. Density measurements were taken by the Archimedean method using 2-propanol alcohol as secondary displacement medium. The average of the three independent measures is reported.
Glass code |
Li2O (mol%) |
MoO3 (mol%) |
B2O3 (mol%) |
Mo-0 |
25 |
0 |
75 |
Mo-1 |
22.5 |
10 |
67.5 |
Mo-2 |
20 |
20 |
60 |
Mo-3 |
17.5 |
30 |
52.5 |
Table 1 Nominal composition of prepared materials
Figure 1 shows the XRD patterns. Samples without or with a low MoO3 content evidence two different deviations from the baseline, above 20º and 40º. The absence of sharp peaks in Mo-0, Mo-1, and Mo-2 allows confirming that samples are glasses. However, Mo-3 shows several sharp peaks due to the formation of crystalline structures. In this light, it is inferred that the formation of a borate glass is facilitated by the incorporation of MoO3 in low quantities only (i.e. less than 30mol%) when small mobile ions like Li+ are present in a proportion around of 20 mol%. Figure 2 displays glass transition temperature as a function of molybdenum oxide content which passes through a minimum value at 10mol% of MoO3. In spite of the fact that Mo-3 is not completely glassy, the Tg of the glass fraction can be identified. Therefore, molar volume (VM) and oxygen packing density (OPD: the number of mol of oxygen atoms per dm3 of glass) calculated from density values are plotted in Figure 3. As a result, it should be assumed that the increment in the content of MoO3 involves a compactness of the glassy matrix. According to the literature, borate glasses are built fundamentally on polyhedra of triangular coordination and the addition of alkali oxides as Li2O forces some of the boron atoms to change from trigonal to tetrahedral coordination23 and, with concentrations of alkali oxide higher than 20%, NBO (no-bridging oxygen) units are formed,24 In the system studied in the present work, the lowest glass transition temperature corresponds to the Mo-1 in which the alkali oxide (Li2O) content is 22.5 mol% and 10 mol% of MoO3. The initial incorporation of MoO3 makes the matrix less stable. Nevertheless, higher MoO3 content (x>0.1) helps to stabilize the matrix and the systems, even in the presence of a moderate alkali oxide concentration, and increases their Tg value. These results explain that the connectivity among boron and molybdenum polyhedra is favorable when the mol MoO3 /B2O3 ratio is not too large where the presence of the alkali oxide causes a minimum perturbation in the 3D connectivity.
From the results in Figure 3 we learn that when a small quantity of molybdenum oxide is incorporated it cuts drastically the glassy matrix (DTg = 75.12 ºC, (Table 2). After that, as the MoO3 concentration increases, the matrix compactness continuously decreases. Molybdenum oxide raises around 20% the glass density value (Table 2) because its molar mass is greater than that of lithium and borate oxides together. However, the molar volume also rises when the molybdenum oxide content augments and as a result, the glassy matrix becomes less compact because of the expansion of its structure. Therefore, the oxygen packing density (OPD) decrease can be interpreted in terms of the formation of a less rigid matrix and resulting in a loosely packed glass compared to the binary one. This reduction agrees with previous results obtained in the case of tellurite glasses.25 A less compact glassy matrix could facilitate the displacement of the mobile ions (Li+). Figure 4 shows the samples infrared spectra. According to the literature, the intensities of the bands from 1000 to 1200cm-1(A) decrease as MoO3 content augments because the substitution of B2O3 by MoO3 diminishes the number of BO3 groups and induces the formation of tetrahedrically coordinated BO4 units (B). 21 Another band lies close to 700cm-1(C), which is attributed to the coupling of MoO3 in chain-like arrangements.26 The studied samples conductivity temperature dependence below the Tg is shown in Figure 5. These data fits well in the general Arrhenius-type formula:
Glass code |
Tg [°C] |
Density [g.cm-3] |
Molar volume [cm3.mol-1 |
OPD [mol.dm<sup>-3</] |
Mo-0 |
509 |
2.23 |
26.72 |
93.55 |
Mo-1 |
434 |
2.42 |
28.13 |
90.66 |
Mo-2 |
512 |
2.62 |
29.19 |
89.06 |
Mo-3 |
539 |
2.77 |
30.69 |
86.35 |
Table 2 Glass transition temperature, density, molar volume and oxygen packing density (OPD).
(1)
Where s0 is the pre-exponential term, Ea: the activation energy for the process involved, T: the absolute temperature and k: the Boltzmann constant. Since only one slope is obtained, we assume that a single conduction process takes place. Despite having of molybdenum in sample Mo-1 to Mo-3, we can assume that the conductivity measured is mostly ionic as it is inferred from the Ea values. Nevertheless, the literature leads us to believe that the conductivity due to the presence of molybdenum in the matrix could be the result of a polaron hopping mechanism. However, if such was the case, it would not be the dominant mechanism as we learn from Figures 6A & Figure 6B. These figures show the conductivity values at 523K, the mean Mo-Mo distance and activation energy as a function of molybdenum oxide fraction. Imre et al.,24 showed that in single-alkali borate glasses the dc conductivity reveals a power-law behavior with an increasing lithium oxide content for x > 0.1.24 I has been shown that in glasses based on vanadium-tellurite oxide, the incorporation of molybdenum causes a dilution of the polaron conductivity.27 Those results are related to relative atomic distances between molybdenum ions. The conductivity decreases upon increasing the oxide molybdenum content might be explained by the presence of MoO3 as network forme.3 In spite of the probable presence of molybdenum ions in different oxidation states (ox.-red. couples), the conductivity could still be ionic and even if the polaronic conductivity was present it would be negligible as it be can inferred from the Arrhenius behavior in Figure 5 and the activation energy values obtained from that. Taking into account that the average distance between two molybdenum ions was estimated, using the experimental density values and elementary chemical considerations, as:
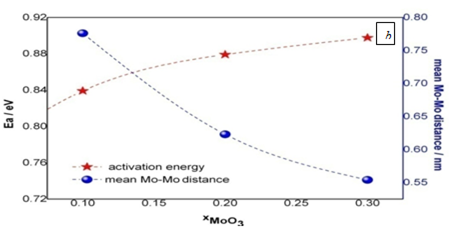

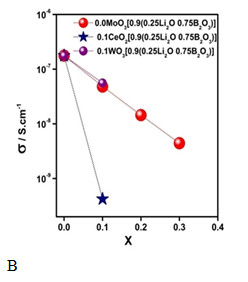
Figure 6 Conductivity at 523 K and activation energy (A), mean Mo-Mo distance and activation energy (B), both as function of xMoO3content.
(2)
where Mm is the sample molar mass, r is the density, NA is Avogadro´s number and x is the molar fraction of the ion of interest, it is evident from the results plotted in Figure 6B that the individual hops of the electron between active sites for polaronic hopping transport (red-ox sites) are getting closer to one another, as xMoO3 increases. However, and against all odds, the Ea of the process increases too. The Anderson-Stuart model leads to interpret and confirm that conductivity, in this case, is dominated by ions and not by polarons.28 Consequently, the Ea obtained from Figure 5 is the barrier that the charge carrier (Li+) has to overcome for hopping and that it raises when xMoO3 increases due to its matrix structure influence. The decrease of the lithium content causes a diminution on the number of free ions (conductivity decreases) and in this B-Mo oxide glass structure the lithium ions are more trapped (Ea augment). Considering that polarons are formed by electron-phonon interaction and that the conduction is by polarons hopping mechanism as was proposed by Austin and Mott, the activation energy (Ea) of the conduction mechanism depends on the average distance between two proper sites for the charge carrier. The results that Figure 6 shows suggest that there is an outstanding positive correlation between activation energy and average distance site separation. It is evident from Figure 7 that the increase of MoO3 concentration does not give polaronic conductivity because a conductivity diminution is evident while the activation energy increases due to the lithium ion content.29 If now we analyze the FTIR results presented in Figure 4, the incorporation of MoO3 in the boron oxide glassy matrix provokes the microscopic changes which tends to form a tetrahedrically connected framework and the more connected the matrix, the less available sites for the lithium ion hopping (i.e. a small number of NBO: non-bridging oxygen) it originates. To reinforce our hypothesis on the interactions among the mobile ions (lithium ions) with molybdenum content, we studied the same composition but replacing the MoO3 by WO3 and by CeO2. Figure 7 shows the comparison of the electrical conductivity of these systems. Rao et al.,16 studied the ESR spectroscopic properties of Li2O -ZnO-B2O3 glass with different contents of MoO3 (from 1 to 5 mol %) prepared by melt quenching. They found that the molybdenum ions, expected to exist mostly in the Mo6+ state when the MoO3 concentration increases beyond 5.0mol. Additionally, IR and optical absorption spectra of paramagnetic ion in such glasses reveal that the site symmetry of the transition metal ion is that of an elongated octahedron.21 B2O3 glasses are good glass formers and in them boron (B3+) ions are present as triangular coordinated polyhedra of oxygen forming the main structural units: BO3 triangles of three member rings connected by B–O–B linkage named boroxol. These rings are planar with a bond length of 1.36A°. BO4 tetrahedral units have a B–O of 1.47A° bond length.30 Borate glasses IR spectra of boroxol rings characteristic absorption is at 806 cm−1.28 Previous structural studies of borate glasses have shown that the addition of a network modifier oxide provokes a conversion of the triangular BO3 units to BO4 tetrahedra incorporated in complex cyclic groups (called diborate, triborate, tetraborate or pentaborate) and a formation of NBO atoms.31,32 Therefore, the absence of the band of boroxol ring (806 cm−1) in a borate glass suggests that the glass consists of randomly connected BO3 and BO4 units.32
The conversion of trigonal BO3 structural units into BO4 tetrahedral unit observed in FTIR absorptions results in the increase of NBOs which facilitates the lithium ion hopping. However, because the Mo6+ cation has a high field strength, it induces an ordering effect on the oxygen ions. Therefore, if a silicate or borosilicate glass modified with molybdenum oxide is combined with alkali oxide, it tends to form crystalline molybdates. It has been shown that the molybdenum in those glassy systems appears as unconnected tetrahedral MoO42- units within an alkali rich glass causing the glassy network depolymerization (i.e., the oxygen atoms of MoO42- units are not bound to silicon or boron atoms). The similarity between the local environment of Mo6+ ions in glasses and in molybdate crystalline phases may explain the low solubility of molybdenum and their tendency to crystallize during the slow cooling of melts or heat treatments. The amount of alkali cations able to form NBO diminishes with the increase of molybdenum concentrations showing that molybdenum keeps alkali ions in its surrounding.17 Therefore, as the glass compositions have mostly the same short-range structure as the crystalline one, it is clear that the intermediate-range structure is strongly dependent on the mobile ions present.33 Figure 8 shows that the permittivity real part increases at every temperature when frequency decreases. The real part of the permittivity goes down at every frequency as the content of MoO3 rises. The comparative diminution is particularly significant at low frequencies due to the lower ion polarization at the interface, not only because the nominal concentration is slightly lower but also because a large number of the mobile ions are trapped around molybdenum charged units. Therefore, in the absence of molybdenum, when temperature rises, the dielectric dispersion shifts towards higher frequencies, the environment around Li+ is changed and it has an influence on the mobility of Li+ ions.
In the present work, we showed that the stability of a borate glass when acting as a host of molybdenum oxide is influenced by the presence of small mobile ions. We studied the interaction between lithium ions with the molybdenum-borate glass which limits molybdenum concentrations before the formation of crystalline structures. We observed in the borate glass x MoO3. (1-x)[0.25Li2O 0.75 B2O3] prepared by the melt quenching technique that the network structure was based on MoO6 octahedral units and on BO3 and BO4 units. However, the charged molybdenum structures interact strongly with those mobile ions present in the matrix and induce order forces that promote the formation of crystalline structures. Such devitrifications are the reason of the aging of the glasses studied here.
Financial support by CONICET (PIP 112 20120100010CO) and Universidad Nacional del Sur (PGI 24/Q078) is gratefully acknowledged. P. E. dP is Fellows of the CONICET. M. E. S. is UNS Researcher, E.C is CIC BsAs Researcher and S.T. and M. A. F. are Researchers Fellow of CONICET – Argentina.

©2018 Cardillo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.