eISSN: 2574-9927


Research Article Volume 6 Issue 3
1Department of Chemistry and Chemical Engineering, Nanjing University, China
2Department of Bioresources Chemical and Materials Engineering, Shaanxi University of Science, China
Correspondence: Jian Hua Zhu, Key Laboratory of Mesoscopic Chemistry of MOE, College of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023,China, Tel 86- 1395195304 9
Received: July 20, 2022 | Published: July 28, 2022
Citation: Sun XD, Yang J, Cao Y, et al. Capturing formaldehyde in cigarette smoke by zeolite and porous sorbents. Material Sci & Eng. 2022;6(3):89-95 DOI: 10.15406/mseij.2022.06.00184
A spectrophotometric method of pararosaniline hydrochloride is reported for rapid determination of formaldehyde (HCHO) content in cigarette smoke in common lab and it has been used to assess the removal of HCHO by zeolites and other porous sorbents conveniently. Through the evaluation in nitrogen flow and cigarette smoke, some porous sorbents were found to exert the attractive performance in capture of HCHO in smoke even in the harsh experiment, which is proven by ISO standard test and provided some candidates for environment protection.
Keywords: formaldehyde, detection, adsorption, zeolite, smoke, environment protection
In order to protect environment and public health, the content of harmful aldehydes in cigarette smoke is becoming more and more concerned. Apart from nitrosamines, polycyclic aromatic hydrocarbons (PAHs) and free radicals, formaldehyde is a well-known poison whose harmfulness is not only limited to smoking but also endangers indoor air. The formaldehyde in the mainstream of cigarette distributes mainly in the gas phase, while 30% of them exists in the particles phase of smoke. Most of formaldehyde is formed by sugar, pectin, protein and some triglycerides in tobacco during the process of smoking, whilst only a small proportion directly transferred from tobacco to smoke.1 Formaldehyde has been listed as a toxic and hazardous air pollutant by The US National Environmental Protection Agency,2 and has also been elevated from a "probable" human carcinogen to a "known" human carcinogen by the International Agency for Research on Cancer (IARC).3 In general, formaldehyde corrodes the cilia of respiratory organs and reduces the removal of excreta from the lungs after inhalation. Biological experiments have confirmed that formaldehyde can cause benign tumors in the nasal cavity of rats and mice. In addition, the non-cancer health effects of formaldehyde include intense irritation of the eyes, skin and respiratory tract and the induction of long-term sensitization.4
Therefore, the systematic, rapid and accurate determination of harmful aldehydes, represented by formaldehyde, in cigarette smoke is necessary and important to reduce the harmfulness of smoking. The usual analytic method of carbonyl compounds is based on the chromatography analysis. The test compound is reacted with 2, 4-dinitrophenylhydrazine by addition-dehydration reaction firstly and then the resulting derivatives are detected by gas chromatography (GC) or high performance liquid chromatography (HPLC).5,6 Due to the limitation of instruments and the special requirement of practical application, we try to establish a spectrophotometric method of pararosaniline hydrochloride for the rapid detection of formaldehyde content in cigarette smoke.
More importantly, how to reduce the content of formaldehyde in cigarette smoke in order to lower the harm of smoking is a topic with both theoretical significance and practical value for tobacco science and environment protection. Activated carbon (AC) filter added into cigarette filters can effectively remove formaldehyde and other aldehydes7, but inevitably affects the taste of cigarette. Laser perforation of filters to increase ventilation also reduces the production of smoking aldehydes, though it is only a physical strategy. Herein, porous materials such as zeolite and mesoporous silica are used to remove formaldehyde in cigarette smoke and compared with the performance of activated carbon. For this purpose, two steps of investigation are accepted. Firstly, the adsorption of aldehydes by porous materials such as zeolite was detected in a simple laboratory system to find effective additive materials. Secondly, zeolite and other porous materials were applied to cigarette filters to assess their actual adsorption performance in a complex system, in order to find the effective sorbent of formaldehyde. More importantly, these performances of zeolites in smoke tested in laboratory system would be compared with those obtained in traditional ISO standard condition to check their reliability, since there was an argument on two experiment systems.8,9 For those non-tobacco scientist, it is necessary to have a simple and quick method to assess the performance of zeolite and other materials in the smoke of tobacco, and the device for collecting cigarette smoke6 is an available choice. However, it is unknown whether the conclusion obtained with this device is consistent with that obtained in ISO standard conditions, which inspired us to do this comparative research for the first time.
Reagents and sorbents
The purity of nitrogen was 99.999%, and other regents such as formaldehyde were AR purity. Zeolites used here were commercially available powder samples. Zeolite NaA, KA and CaA were commercially available powder products from Shanghai Zeolite (China). Zeolite CsA was obtained by ion exchange of KA with 0.2 mol L-1 CsCl solution for 3 times.10 Hβ zeolite with a Si/Al ratio of 14 was obtained from BASF. MCM-22 was from China University of Petroleum. Zeolite NaX was from Nanjing Inorganic Chemical Plant (China), while NaY and NaZSM-5 were purchased from Catalyst Plant of Nankai University (China). HZSM-5 zeolite was prepared by conventional ion exchange method with aqueous solution of NH4NO3, at the solid/solution ratio of 1:15, performing at 353 K for 2 h and repeating for 6 times. The obtained sample was washed, filtrated and dried at 393 K followed by calcination at 823 K. HY and HX samples were acquired by the similar process except the calcination of NH4Y or NH4X at 773 K. CAS-1 sorbent was a porous material with the pore size close to that of zeolite KA11, and it was provided by Taiyuan University of Technology. Both mesoporous silica SBA-15 and MCM-41 were synthesized in our laboratory according to recipes in the literature.12,13
The coconut shell-activated Carbon (AC, 20-40 mesh) was obtained from Chemviron Carbon, and amorphous silica gel (referred as to silica, 100-200 mesh) was purchased from Qingdao Ocean Chemical (China). Sorbent of γ-alumina was commercially available powder. The cigarettes made from British flue-cured tobacco were purchased from market. Preparation and calibration of reagent solution, along with the establishment of a concentration-absorbance standard curve of formaldehyde were described in the section of Appendix as shown in Figure S1.
Evaluation on the adsorption of formaldehyde in gas flow
To detect the HCHO content of gas flow, all flue gas was passed through water to be absorbed, and the obtained solution was then washed with dichloromethane to remove the interferences in the aqueous phase. Subsequently, formaldehyde in the solution reacted with sulfur dioxide and parafuchsin hydrochloride (also known as pararosiniline hydrochloride, whose molecular formula is C19H18ClN3) to produce a red-purple complex (Figure 1), and its concentration was determined by colorimetric method.14
Figure 2 described the experimental apparatus and reagent containing test tube, color bottle, U-shaped tube, quartz sample tube, water bath, formaldehyde solution, anhydrous calcium chloride, zeolite and other porous materials, as well as distilled water. Practically, 25 mL 37-40% (W/V) formaldehyde solution was put into a 100 mL volumetric flask and brought to volume with water, and 10 mL of the solution was then taken for detection in a 303 K water bath. Carrier gas was usually nitrogen and its flow rate was 30 mL min-1. 10 g of anhydrous calcium chloride was used to remove moisture in the gas, while 40 mg of granular sorbent sample (20-40 meshes) was placed in a quartz tube to capture the formaldehyde passed. The residual formaldehyde in gas flow was then absorbed in an ice water bath for 30 min, in which the flask contains 42 mL distilled water to trap the formaldehyde. Finally, 5 mL 0.2% pararosaniline hydrochloride and 3 mL 0.1% sodium bisulfite solution were added into the flask for color reaction at a constant temperature of 298 K for 90 min. The amount of residual formaldehyde in flask trapper was determined by spectrophotometry (Figure 2). On the other hand, same method was used to measure the blank formaldehyde content without sorbent, in order to assess the performance of sorbent to adsorb formaldehyde difference subtraction.

Figure 2 Instrument of trapping formaldehyde in gas flow by porous materials and the block diagram about the concentration detect of HCHO.
Detection of formaldehyde content in cigarette smoke
The device for collecting the formaldehyde in cigarette smoke6 is shown in Figure 3, in which 168 mL aqueous solution (containing 8 mL 1% sulfamic acid) was evenly divided into four tubes to absorb the formaldehyde produced by the combustion of 2 cigarettes. 40 mg zeolite, in 20-40 meshes, was carefully added into the filter to replace part of cellulose matrix with a same volume. Two cigarettes were smoked in the glass-made chamber, and mainstream was pulled through the 168 aqueous solutions. All the collecting liquid of the four connecting pipes were transferred to a 250 mL separating funnel and the pipes was washed with 60 mL dichloromethane. Finally the 60 mL washing dichloromethane was also transferred to the separating funnel. After a minute of intense oscillation, the dichloromethane layer was left standing for stratification, and then the dichloromethane layer was discarded. The water layer was moved to the 250 mL measuring bottle, and brought to volume with water to form the sample solution. Taking 20.0 mL of the sample solution and added into a 25 mL volumetric flask, and then added 1.5 mL sodium bisulfite solution and 2.5 mL parfuchsin hydrochloride solution. After this solution was mixed fully, it was brought to volume with distilled water. The absorbance was measured with spectrophotometer after 90 minutes. Blank solution without sample solution was prepared in the same procedure as control.
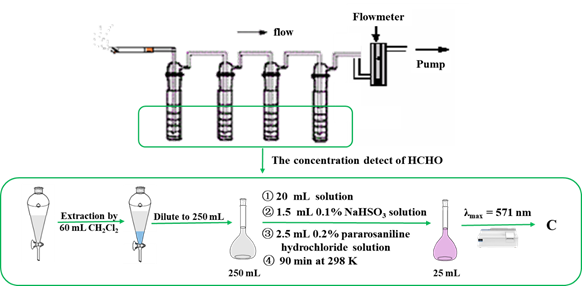
Figure 3 The apparatus for the mainstream smoke experiment and the block diagram about the concentration detect of HCHO.
According to the measured absorbance data, the actual concentration C of the test fluid could be found from the standard curve, and thus the formaldehyde content of smoke could be calculated using the following formula:
Formaldehyde (microgram/branch) =
Where C: formaldehyde concentration in the test solution obtained from the standard curve; V1: the volume of water phase after extraction by dichloromethane; V2: the volume of V1 liquid product moved for color rendering; V3: the volume of color solution. Usually, the data of V1, V2 and V3 was 250 mL, 20 mL and 25 mL, respectively, and N was 2.
Removal of formaldehyde in smoke by adding porous sorbent into cigarette filter
The porous material samples were added to the middle part of the cigarette tip at 40 mg cig-1 and balanced for more than 48 hours. The controls are empty (no sample was added in the middle of filter but left a space). Cigarettes are smoked by single-hole smoking machine produced by Heinrich Burghart. Each sample was burned and smoked with three sample cigarettes. The mainstream smoke generated was collected by tail gas bag and injected into GC-MS instrument for analysis and detection.15
Establishment of standard curves
The formaldehyde solution with a concentration of 0.2, 1.0 and 5.0 mg mL-1, respectively, was selected to react with the solution of parfuchsine hydrochloride and scanned in the range of 400-700 nm. As the obtained spectrum shown in Figure 4a, the maximum absorption wavelength of the solution was determined at 571 nm. Therefore, UV absorption wavelength of 571 nm was chosen to determine the concentration of formaldehyde solution with UV spectrophotometry in practice.
The formaldehyde solution with a relatively high concentration in the range of 0-5.0 mg L-1 had a good linear relationship with the corresponding absorbance (Figure 4b). The repetitions tests for 5 times proved that the spectrophotometric detection method had a very good reproducibility (Figure 4b), and its linear regression equation was A = 0.39475C +0.124, where the unit of C was μg mL-1. In addition, the correlation coefficient R was 0.9999.
Zeolite samples can absorb efficiently the formaldehyde in gas flow, as described later, so that only a few formaldehyde will be collected in the ice water bath (Figure 2). For this reason, it is necessary to investigate the linear relationship of the standard curve with these diluted formaldehyde solutions (0.2-0.8 mg mL-1). The reproducibility of the standard curve in the low concentration range of formaldehyde, as displayed in Figure 4c, was not as good as that at high concentration (Figure 4b). Nonetheless, the relative standard deviation (RSD) was still less than 3%, indicating its usefulness for determination of formaldehyde in diluted solution. The linear regression equation was A = 0.382C +0.132, and the correlation coefficient R could also reached 0.9995. Through comparison of Figure 4b and Figure 4c, it was found that the slope of the standard curve increases slightly when the concentration of sample solution increases. However, the data points still showed a linear relationship on the whole, even in the different concentration ranges. In practical application, it is better to pre-estimate the concentration range of formaldehyde samples in advance, and then choose an appropriate standard curve to analyze them.
In combination with the standard curves of normal and low concentrations (Figure 4b and Figure 4c), it can be seen that the absorbance was linearly related to formaldehyde content in the range of 0-5 μg mL-1 (Figure 4d), and its regression equation was A = 0.3944C + 0.124, which is basically consistent with the regression equation of the standard curve of high concentration, with a correlation coefficient R=0.9997.
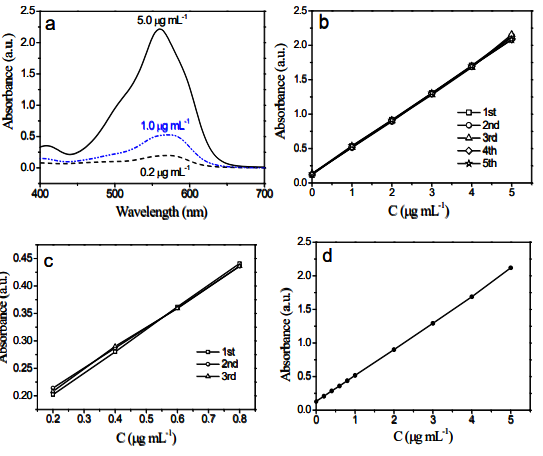
Figure 4 (a) detection of formaldehyde with parfuchsin hydrochloride and UV scanning. (b) Absorbance curve at high HCHO concentration. (c) Absorbance curve at low HCHO concentration. (d) The standard absorbance curve via HCHO concentration.
Adsorption of formaldehyde with porous materials
As described previously, the mass fraction of formaldehyde solution used in the experiment of Figure 2 was about 10% (w/v). In the 30 min of nitrogen flow purging, the total amount of formaldehyde passed through was detected as 48.6~55.0 μg. This value was close to that in the mainstream smoke of one cigarette.16 Table 1 lists the structural properties and performance of zeolites and other porous materials in adsorption of formaldehyde. With the exception of zeolite CsA, KA and CS-1 samples, most of zeolites showed a good adsorption of formaldehyde in nitrogen flow, and their removal were 85% or more. The low activity of zeolite CsA and KA and CS-1 was related to their small micropore size of about 0.3 nm10,11, which limited the entry and diffusion of adsorbate.17
|
Sample |
Pore size |
Pore volume |
Surface area |
Si/Al |
Removal in gas flow (%) |
Removal In mainstream smoke (%) |
|
(nm) |
(Cm3 g-1) |
(m2 g-1) |
||||
|
CaA |
0.49 |
0.21 |
636 |
1 |
91.4 |
9.9 |
|
NaA |
0.39 |
0.28 |
800 |
1 |
86.1 |
4.1 |
|
KA |
0.33 |
0.24 |
740 |
1 |
59.2 |
- |
|
CsA |
0.21 |
un |
un |
1 |
25.7 |
6.5 |
|
CS-1 |
~0.30 |
un |
un |
- |
20.5 |
15 |
|
NaZSM-5 |
0.54×0.56 |
0.11 |
354 |
12.5 |
98.2 |
6.5 |
|
HZSM-5 |
0.54×0.56 |
0.1 |
346 |
12.5 |
80.9 |
10.5 |
|
MCM-22 |
0.40×0.55 |
0.36 |
481 |
10 |
88.6 |
32 |
|
HMCM-22 |
0.40×0.55 |
un a |
un |
10 |
99.3 |
25.2 |
|
Nab |
0.66×0.67 |
0.8 |
643 |
14.2 |
98.2 |
4.8 |
|
Hb |
0.66×0.67 |
0.8 |
607 |
14.2 |
98.8 |
6.8 |
|
NaX |
0.74 |
0.3 |
900 |
1.24 |
93.8 |
7.5 |
|
HX |
0.74 |
un |
un |
1.24 |
92.3 |
16.3 |
|
NaY |
0.74 |
0.31 |
766 |
2.86 |
99 |
8.5 |
|
HY |
0.74 |
0.3 |
550 |
2.86 |
91.8 |
9.5 |
|
MCM-41 |
4 |
1.12 |
1342 |
- |
99.2 |
11.9 |
|
SBA-15 |
8.4 |
1.18 |
918 |
- |
91.6 |
32.3 |
|
Alumina |
2.0-10.0 |
0.42 |
209 |
- |
88.6 |
- |
|
Silica |
8.0-10.0 |
0.8 |
350 |
- |
97.9 |
22.8 |
|
AC |
1.57 b |
0.49 |
921 |
- |
97 |
15 |
Table 1 Adsorption of HCHO in gaseous phase by zeolites and other sorbents
However, the length of H-H or H-O bond in the HCHO molecule is theoretically about 0.19 or 0.20 nm, smaller than the pore size of KA zeolite (about 0.3 nm) hence geometric matching seems not the sole reason to cause its poor adsorption. CS-1 sorbent had a fibrous morphology14 but it made no contribution to trap the formaldehyde in gas flow. As the pore size of zeolite increased to 0.5 nm or more, the overwhelming majority of formaldehyde was captured in gas phase (Table 1), coincided with that reported with lower HCOH vapor pressure.18 Formaldehyde is a neutral compound hence these large-micro porous zeolite, X, Y and β type, with sodium cations or proton had the similar adsorptive performance, with exception of ZSM-5 and MCM-22 zeolite whose pore size was around 0.5 nm (Table 1). Siliceous mesoporous sorbent MCM-41 and SBA-15 without metal cations also had high adsorption capacity, implying the major role played by the wide pore opening instead of cation in the formaldehyde adsorption. Similarly, amorphous alumina and silica along with activated carbon (AC) also exhibited a high efficiency to trap formaldehyde in nitrogen gas flow (Table 1), indicating their potential application in purifying indoor air.19
The performance of zeolite and other samples in capture of the formaldehyde in mainstream smoke also was assessed using our lab’s own method as shown in Table 1. Adsorption of formaldehyde in cigarette smoke is a nightmare of sorbents because the smoke contains hundreds of chemical compounds20 that seriously disturb the adsorption, which has been verified in nitrosamines capture.11,12,17 The removal of formaldehyde by activated carbon (AC) was only 15% (Table 1), much lower than its performance in nitrogen flow (97%), while the champion among zeolites, MCM-22 could capture 32%, only 36% of that in N2 flow (88.6%, Table 1). Nonetheless, the MCM-22 with proton instead trapped less (25%) formaldehyde. These large-micro porous zeolites X, Y and β whose removal of HCOH exceeded 90% in nitrogen flow, adsorbed 16% or less in the smoke. For those zeolites with middle or small micro pores such as ZSM-5 and A, they captured 10% or less formaldehyde in mainstream smoke (Table 1), which reveals the powerless of ordered micro porous structure to adsorb formaldehyde in such complex system, as that reported in capture of tobacco specific nitrosamines (TSNA) in tobacco smoke.21
The enough wide mesopores were beneficial for the formidable adsorption, since SBA-15 trapped 32% of HCHO in smoke but MCM-41 adsorbed 11% (Table 1). Amorphous coarse pore silica showed a fair performance, removing 22% of formaldehyde in smoke and implying the importance of complex pore structure in the complex system.21 In addition, the special morphology of sorbent was proven to be important for such adsorption, CS-1 with fibrous morphology11 trapped 15% of HCHO in smoke, similar to that by AC, while the MCM-22 sorbent with "rose-like" morphology12 captured 32% though its pore size is close to that of ZSM-5 zeolite. The integral morphology of sorbent plays an important role to elevate the efficiency of adsorption in the complex system such as tobacco smoke,21 which is beneficial to intercept and trap the target in smoke.11
We turn now to Figure 5 that lists the removal of formaldehyde in mainstream smoke by the porous additive in cigarette filter detected with different methods. One method used LC-MS /MS instrument in the Research Center of British American Tobacco (abbreviated as BAT), and another adopted the UV spectrophotometry in our laboratory (abbreviated as NJU). It is obvious that the formaldehyde removal measured by LC-MS/MS method was mostly higher than that by UV method (Figure 5), with the exception of NaA zeolite, and among them the difference in the data of NaZSM-5, HZSM-5, NaY zeolites and MCM-41 was particularly obvious. Their removal of HCHO detected by UV method was 6.5%, 10.5%, 8.5% and 11.9% (Table 1), while the values of LC-MS/MS were 26%, 37%, 14% and 60% (Figure 5). This is not surprising since the sample cigarette was smoked in different ways. The ISO standard suction of cigarette was applied in the test of BAT in which 2 s suction per mouth was set with the volume of 35 mL, and the interval between each mouth was 58 s hence only part of the formaldehyde in smoke would contact with filter. However, the sample cigarette was sucked continually after lighted in the instrument of our laboratory, with an air flow rate of 3 L min-1 that equals 50 mL sec-1 and exceeded that of ISO test (17.5 mL sec-1). Moreover, since the cigarette was smoked continually, in which the yield of carbonyl compounds were 3 to 7 times higher,8 and the total formaldehyde (about 116.5 μg cig-1, more than that of ISO test, about 60 μg cig-1)1 passed through the sorbent with a fast flow rate. Consequently, it is very difficult for the porous materials added into the filter to adsorb the formaldehyde in smoke. For a quite long time, there was an argument on the two smoking manners8, because the ISO standard suction of cigarette was close to actual cigarette smoking so the detected amount of hazardous substance in smoke was close that smoker contacted. However, the continually sucked smoking method can be applied to assess the adsorption of zeolite in smoke, since it was rather vigorous because the entire smoke produced from a cigarette in a short duration of time totally passed through the sorbent.9,22 Even so, two different methods confirm the efficiency of some zeolites to trap the formaldehyde in smoke, coincided with that of nitrosamines trapped in smoke,12 and among them the adsorption performance of MCM-22 (40%, Figure 5), HZSM-5 (37%) zeolites and SBA-15 (38%) and MCM-41 (60%) mesoporous silica was higher than that of activated carbon (30%) (AC, Figure 5). These results provide some candidates to control the pollution caused by formaldehyde in cigarette smoke, along with a rapid evaluation method.
Pararosaniline hydrochloride spectrophotometric can be used to detect formaldehyde content in cigarette smoke conveniently Many zeolites and mesoporous silicon sorbents are able to trap the formaldehyde in nitrogen gas flow efficiently, indicating their potential application in purifying indoor air. Some of them can also effectively reduce the formaldehyde content in mainstream smoke of cigarette, reducing the harm of smoking. Both pore structure and morphology of sorbent affect its performance in the capture of formaldehyde in tobacco smoke. Presence of silicon is beneficial to adsorb formaldehyde. Amorphous silica removed 22% of formaldehyde in the smoke in the harsh smoking test, while mesoporous material SBA-15 trapped 32%, providing a potential sorbent to control the pollution of formaldehyde.
NNSF of China (No. 21673113), and Analysis Center of Nanjing University financially supported this research. The authors thank BAT for their co-operation.
Dr. Xiao Dan Sun (xdsun@sust.edu.cn): Adsorption; Draft preparation.
Dr. Jing Yang (yangj@sh.tobacco.com.cn): Experiment of adsorption.
Dr. Yi Cao (caoyi@jszygs.com.cn): ISO standard condition test.
Professor Dr. Jian Hua Zhu (jhzhu@nju.edu.cn): Writing- Reviewing and Editing.
Professor Dr. Wang is a famous chemical expert and he has studied novel ordered mesoporous materials and adsorption for many years.
Professor Dr. Chen spent a long time to adsorption and catalysis, and design and prepared some new functional materials for chemical reactions to elevate the efficiency of catalysis. Recently he changed his interest on environment protection.
Professor Dong has a strong research background on selective adsorption, environment protection and new nanometer materials.
The reason: The research of Professor Dr. Wu covers such diverse topics as: (a) adsorption at the gas/solid and liquid/solid interfaces, (b) elaboration of advanced adsorbents, catalysts and other materials, (f) characterization of zeolites, silica, alumina and other porous materials, in order to enrich information about surface and structural properties of these materials.
Preparation and calibration of reagent solution
For preparation of 0.1000 mol L-1 potassium iodate standard solution [C (1/6 KIO3)=0.1000 mol L-1], 3.5668 g potassium iodate (first grade) was accurately weighed and dried for 2 hours at 378 K. It was completely dissolved in distilled water and transferred to a 1000 mL volumetric flask, diluted to scale and shaken well.
The KIO3 reference material was used to calibrate the concentration of Na2S2O3 solution. Practically, a certain amount of reference material was weighed, reacted with excess KI in acidic solution, and the I2 elemental precipitated by Na2S2O3 solution was titrated with starch as indicator. The related equation is as follows:
The optimal reaction conditions of KIO3 and KI were determined as follows: (1) The higher the acidity of the solution, the faster the reaction speed, but when the acidity is too high, I- is easy to be oxidized by O2 in the air, so the suitable acidity is generally 0.2 ~ 0.4 mol L-1. (2) When KIO3 interacts with KI, it is unnecessary to place a time. In contrary, titration should be carried out in time. (3) The KI solution used should not contain KIO3 or I2. If the KI solution is yellow, it should be titrated with Na2S2O3 solution to colorless before use. Also, if the solution soon changes to the blue color of I2 starch after titration reaches the end point, it indicates that the incomplete reaction of KI and another solution should be taken for re-calibration.
To prepare 0.5% starch solution, 0.5 g soluble starch was added into 5 mL distilled water to make a paste, then 100 mL boiling water was added, and boiled for 2-3 minutes until the solution was transparent. After cooling, this solution was added 0.1 g salicylic acid for preservation (enzyme inhibition and antibacterial effect, as a preservative). For preparation of 0.1 mol L-1 sodium thiosulfate standard solution, 25 g sodium thiosulfate (Na2S2O3·5H2O) was dissolve in newly boiled cooling water, then 0.2 g sodium carbonate was added. This solution was diluted to 1000 mL while any turbidity should be filtered, and then stored in a brown bottle. After storage of one week, the concentration of this solution was calibrated using the following method.
Taking a precise amount of 25.00 mL 1.000 mol L-1 potassium iodate standard solution and putting it to a 250 mL iodine measuring flask, followed by addition of 75 mL fresh boiled and cooled water, 3 g potassium iodide and 10 mL glacial acetic acid, respectively. The solution was shaken well and then placed in dark for 3 minutes, then the precipitated iodine was titrated with sodium thiosulfate standard solution to light yellow. After adding 1 mL 0.5% starch solution, the solution turned blue, and titration was continued until the blue just faded, which was the end point. If the volume of sodium thiosulfate solution used was recorded as V (mL), the concentration of sodium thiosulfate solution (CSTS) could be calculated by the following formula:
CSTS =
Na2S2O3 is not a reference substance therefore it cannot be directly formulated as a standard solution. The prepared Na2S2O3 solution is unstable and easy to decompose, because the following reactions occur under the action of microorganisms in water, CO2 and O2 in air:
In addition, trace amount of Cu2+ or Fe3+ in water can also promote the decomposition of Na2S2O3 solution. Therefore, when preparing Na2S2O3 solution, it is necessary to use freshly boiled (in order to remove CO2 and kill bacteria) and cooled pure water, and add a small amount of Na2S2O3 to make the solution weakly alkaline to inhibit bacterial growth. Even the solution prepared in this way is still not suitable for long-term storage and thus needs to be re-calibrated after a period of use. If the solution was found to become muddy or sulfur precipitation, it should be filtered and then calibrated.
To prepare 0.1 mol L-1 iodine solution [C(1/2 I2)=0.1 mol L-1], 30 g potassium iodide was dissolved in 25 mL distilled water, then 12.7 g iodine added. After solids dissolved completely, this solution was diluted to 1000 mL with water, then moved into a brown bottle and store in dark. To prepare 0.2% hydrochloric acid parfuchsin solution, taking 0.1000 g of parfuchsin hydrochloric acid solution and dissolve it in 188 mL solution containing 15 mL concentrated hydrochloric acid (12 mol L-1) and dilute it to 250 mL. To prepare 0.1% (W/V) NaHSO3 solution, 0.50g NaHSO3 solid was dissolved in water and the solution was added into a 500 mL brown volumetric flask with constant volume. Even so, it only lasted a week. Amino sulfonic acid was used here to remove the interference of nitrogen oxides.
Drawing a concentration-absorbance standard curve of formaldehyde
For preparation of standard solution of formaldehyde, taking 1.4 mL of formaldehyde with a content of 36~38% and putting it into a 500 mL volumetric flask and diluted with water to scale. This solution could be stored for 3 months and it contained about 1 mg of formaldehyde in 1 mL. However, its exact concentration needs to be determined by the following iodimetry method. Firstly, 20 mL of formaldehyde diluent was put into a 250 mL iodine flask, followed by addition of 20 mL 0.1 mol L-1 iodine standard solution and 15 mL 1 mol L-1 NaOH solution, and then placed for 15 min. Next, 25 mL 0.5 mol l-1 sulfuric acid solution was added and placed for another 15min followed by titration with 0.1000 mol L-1 sodium thiosulfate standard solution. When this solution was pale yellow, adding 1 mL0.5% starch solution to turn it blue and continuing to drop until the blue just fades, that is, the end point. The volume V2 (mL) of sodium thiosulfate standard solution consumed in titration was recorded. At the same time, the reagent was used for blank titration within the procedure same as above, and the volume V1 (mL) of sodium thiosulfate standard solution consumed was also recorded. The sample titration and blank titration were repeated twice, and the volume of sodium thiosulfate used for two titrations was no more than 0.05 mL. The concentration of formaldehyde solution (X) is calculated by the following formula:
X (mg mL-1) =
Where M -- concentration of sodium thiosulfate;
15 -- 1/2 the molar mass of formaldehyde.
20 -- Milliliters of the standard volume of formaldehyde stored solution taken during calibration.
For preparation of 25 μg mL-1 formaldehyde standard solution, putting 25/X mL of the above solution into a 1000 mL volumetric flask, and diluted it with distilled water to the scale, then the concentration of formaldehyde in the diluted solution was 25 μg mL-1. It was placed for 30 minutes to prepare different standard solution for analysis and such standard solution was stable for 24 hours. Taking the diluted solution 0, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 10.0, 12.0 mL and put them into ten 25 mL volumetric bottles, respectively, then dilute them with water to 20 mL. Then, 1.5 mL sodium bisulfite solution and 2.5 mL parfuchsin hydrochloric acid solution were added to each volumetric flask. After fully mixing, water was added to scale (the concentration of formaldehyde in this series of solutions is 0.00, 1.00, 2.00, 3.00, 4.00, 5.00, 6.0, 8.0, 10.0, 12.0 μg mL-1, respectively). After well mixing, these solutions were heated in 298 K water bath for 90 min. Three samples (0.00, 1.00, 12.00 μg mL-1) were selected for full UV scanning to measure the maximum absorption wavelength. A concentration-absorbance standard curve was drawn based on the concentration of the standard solution (in μg mL-1) and the measured absorbance data.
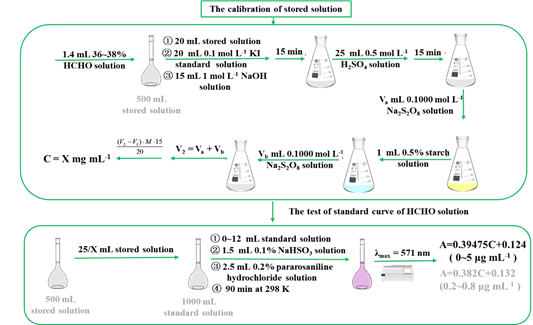
Figure S1 The block diagram about the calibration of stored solution and the test of standard curve of HCHO solution.
Graphic Abstract
A spectrophotometric method of pararosaniline hydrochloride is reported for rapid determination of formaldehyde (HCHO) content in cigarette smoke, and zeolite especially MCM-22 and SBA-15 exhibited better removal than active carbon (AC).

©2022 Sun, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.