eISSN: 2574-9927


Research Article Volume 3 Issue 5
1Programa de Pos Graduacao Interunidades Bioengenharia, Brazil
2Universidade Estadual Paulista, Brazil
Correspondence: Paula RF de S Moraes, Programa de Pos Graduacao Interunidades Bioengenharia – EESC/FMRP/IQSC – USP, Av. Trabalhados Sao-carlense, 400, Arnold Schimidt, CEP:13566-590, Sao Carlos, SP, Brazil
Received: August 31, 2019 | Published: October 1, 2019
Citation: Moraes PRDS, Ribeiro SJL, Gaspar AMM. Bacterial cellulose/phytotherapic hydrogels as dressings for wound healing. Material Sci & Eng. 2019;3(5):162-170. DOI: 10.15406/mseij.2019.03.00108
Healing is a complex process that involves cellular and biochemical events, and several medicines have been used to shorten healing time and avoid aesthetic damage. The development of an objective, non-invasive, and in-vivo examination method is urgently demanded towards helping the determination of the depth and degree of wound healing. This manuscript reports on a comparison of two wound dressings based on bacterial cellulose/phytoterapic (Calendula officinalis and Jacaranda caroba) hydrogel applied to rat dorsum with bacterial cellulose hydrogel and untreated wound. The hydrogels were characterized by Scanning Electron Microscopy, Thermogravimetric Analysis and Fourier transformed - Infrared spectroscopy, and the results showed they are biocompatible and easy to attach, keep the site moist and do not damage the granulation tissue. According to the in vivo test, Bacterial Cellulose/Calendula officinalis hydrogel promoted a better wound healing and statistically significant differences in the tissue repair between treatments on the 3rd and 7th days (critical periods) after surgery. The results of the histological evaluation demonstrated a statistically significant difference for tissue inflammatory reaction between the treatments in the 3-day period. A statistically significant difference between the treatments in the evaluation of the quality, quantity and orientation of the collagen fibers was verified in the 7-day period. The hydrogels (especially bacterial cellulose with Calendula officinalis) yielded satisfactory results and, therefore, can contribute to the tissue repair of rat skin wounds.
Keywords: bacterial cellulose, Calendula officinalis, Jacaranda caroba, hydrogel, wound dressing, tissue repair
Skin, the largest organ of the human body, is formed mainly by epidermis and dermis and its main functions are assurance of mechanical protection and prevention against contamination.1,2 Wound healing begins from a skin-barrier disruption and is divided into inflammatory, proliferative, and maturation phases. The former consists in the recruitment of leukocytes to the site of the lesion. In the proliferative phase, the migration and proliferation of keratinocytes, fibroblasts, and endothelial cells results in reepithelization and formation of granulation tissue with a large quantity of type III collagen. Finally, in the maturation phase, most type III collagen fibers are substituted by type I fibers and the excess collagen is degraded by proteolytic enzymes that promote tissue remodeling. Despite some recent advances in the understanding of such basic processes, wound healing disorders continue to cause diseases and even death.3 Dressings play a substantial role in the conglutination of certain types of open wounds (e.g. traumatic, thermal or chronic wounds), since the moist, warm and nutritious environment of wound beds provides an ideal condition for microbial growth. The wound healing process can interfere with bacterial colonization and subsequent infection, which may cause an excessive and prolonged inflammatory response from the host tissues. The nature of lesions, patient’s physiologic state, wound degree of infection and contamination and other disease processes can interfere with the cutaneous wound healing.4 The basic requirement for a material to be used for tissue engineering purposes is biocompatibility. Over the past two decades, significant advances have been made regarding the development of biodegradable polymers and biodegradability is one of the most important properties, since the scaffold should degrade with time and be replaced with newly regenerated tissues.5
In comparison with other biopolymers, e.g., collagen, chitosan and gelatin, bacterial cellulose (BC) displays excellent biological properties for tissue regeneration, mainly for the treatment of chronic and burn wounds.6,7 It is produced as never-dried membranes free of lignin and hemicelluloses, which show high elastic modulus and tensile strength when wet.8 Since the 80s and 90s, BC-based dressings (as Biofill® (Fibrocel, Brazil)) have been used as temporary dressings for the treatment of skin wounds mainly in burns, graft and chronic ulcers.6,9‒11 The use of medicines and plants for the treatment of several diseases, even for favoring wound healing, is an ancient practice. Calendula officinalis (CO) was used in the Middle Ages for wound healing and treatment of inflammatory skin diseases. In Germany, the topical use of marigold preparations is still very popular. C. offcinalis is a medicinal plant with diverse biological activities.12 Its experimental topical use in rats as a cream preparation showed both anti-inflammatory and wound-healing effects13 and faradiol esters were identified as the most potent single substances in the marigold extract.14Jacaranda leaves macerated in alcohol (cachaça) have been widely applied for cicatrization and ingestion in treatments of ulcers.15,16 In Brazil, the inhabitants of Vale do Ribeira, São Paulo, have employed this species for combating syphilis, wounds and ulcers.15 Despite the existence of modern and advanced technologies in the pharmaceutical industry, market products that stimulate the wound repair process are still limited. This study evaluated the topical effect of two hydrogels based on bacterial cellulose and two phytoterapics (Calendula officinalis (CO) and Jacaranda caroba (JC)) on the healing process of rat skin wounds and the organization of collagen fibers in the injured tissue.
Hydroalcoholic tincture (20% extract in 70% ethanol) of Calendula officinalis flowers was commercially obtained from Calêndula Pharmacy (São Carlos, SP, Brazil). Dried extract of J. caroba leaves, collected in Minas Gerais state, Brazil, was obtained from Universidade Federal de Minas Gerais and Jacaranda caroba tincture (20% extract in 70% ethanol) was prepared at the Institute of Chemistry from São Carlos, at Universidade de São Paulo.
High-performance liquid chromatography (HPLC)
The phytochemical profile of the tinctures was determined by a Shimadzu (Kyoto, Japan) liquid chromatography system equipped with an LC-10 AT VP solvent pump unit and an SPD-10A VP UV-Visible detector operating at 340nm. Samples were manually injected by a Rheodyne injector (20µL loop) and the compounds were separated in a C18 Hypersyl BDS-CPS cyano column (250mm x 4.6mm, 5µm) (Thermo Electron Corporation, USA). The mobile phase was methanol-water (20:80, v/v) for J. caroba and (25:85, v/v) for C. officinalis at a 1mL/min flow rate for 60min, and data were collected by LC Real Time Analysis software. All procedures were conducted at the Institute of Chemistry from São Carlos, at Universidade de São Paulo.
Preparation of hydrogels
Bacterial cellulose (BC) was obtained from cultures of Gluconacetobacter hansenii (ATCC 23769 strain). G. hansenii bacteria were cultivated in 100mL flasks with a 20 mL static culture medium for 120h at 28°C. The nutrient medium contained 2wt% glucose, 0.5wt% peptone, 0.5wt% yeast extract, 0.27wt% disodium hydrogen phosphate, and 0.115wt% citric acid. Bacterial cellulose pellicles formed on the air/liquid interface were harvested and purified by immersion in a 2wt% NaOH solution at 80°C for 1h. Subsequently, they were immersed in a 1 wt%NaClO solution for 30 min, washed with deionized water and sterilized by autoclave at 120°C for 15min. BC/CO and BC/JC hydrogels were prepared with sterilized instruments. Never-dried BC membranes were triturated in an aqueous medium by an Ultra Turrax T18 Ika high-speed disperser element. The BC particles suspension was sieved (37mesh) for the removal of excess water. The BC pulp was used in the preparation of the hydrogels. 0.2wt% of Nipagin® (Henri Farma, Brazil) were solubilized in 5wt% propyleneglycol (Pharma Nostra, Brazil) under orbital shaking at 50°C. The mixture was poured into a beaker containing 10wt% of bacterial cellulose pulp under mechanical stirring at RT. 3wt% of Natrosol (Hydroxyethylcellulose) (Pharma Nostra, Brazil) were slowly added under shaking until the obtaining of gel consistency at RT. 10wt% of CO and JC tincture and deionized water were added to each treatment. The hydrogels were kept under shaking for 3min at RT and then stored in a sterile vial.
Physicochemical characterization
Scanning electron microscopy (SEM): Samples of hydrogels were freeze-dried (−40 to−50°C) at constant weight prior to examination by scanning electron microscopy. SEM images were obtained by a JEOL/EO - JSM–6610 scanning electron microscope for the analysis of changes in the micro fibrillar structure in lyophilized samples coated with a 10nm carbon layer by a metallizer Balsers model CDS 050. The morphology was observed at 15kV accelerating voltage.
Thermogravimetric analysis (TG): The thermogravimetric curves of the samples were recorded by a TA SDT 2960 from TA Instruments Co. All samples were heated in alumina pans at a 25 °C to 800 °C temperature range under nitrogen atmosphere (100 mL min-1 flow rate) and 10 °C min-1 heating rate.
Fourier transform infrared (FT-IR): Fourier transform infrared (FT-IR) spectra were obtained with lyophilized powdered samples by a Perkin Elmer Spectrum 2000 spectrophotometer. Pellets were prepared from mixtures of the samples and KBr (1:100 in weight). Sixty-four scans were accumulated at 1cm-1 resolution in the 4000 to 400cm-1
Cytotoxicity and cell viability: The essay was performed at the Department of Physiology of the Federal University of São Carlos under the supervision of PhD Caroline Faria Bellani.
Cell culture: L929 mouse-derived fibroblasts were maintained in a DMEM medium (Life Technologies™) supplemented with 10% fetal bovine serum (Vitrocell®), penicillin-streptomycin (1%) and amphotericin b (2mg/L).
Preparation of the hydrogel extracts
The hydrogel samples were dissolved in a 10% (v/v) DMEM culture medium for 48 hours under magnetic stirring. The extracts were kept under refrigeration at 8°C and applied to the respective groups of cells to be studied.
Proliferation and cell viability
The AlamarBlue® (Life Technologies™) assay checked the influence of the hydrogels on the cell growth and viability over time. AlamarBlue® is a reagent with an oxidation-reduction indicator (REDOX) that both fluoresces and changes its color in response to chemical reduction, which is the result of cell growth in a culture medium. A continuous cell growth maintains a reduced environment (fluorescent, red), whereas the inhibition of cell growth maintains an oxidized (non-fluorescent, blue) environment. The AlamarBlue® assay quantitatively measures the relative proliferation and viability or cytotoxicity of various human, animal, bacterial or fungal cell lines.17 L929 fibroblasts were counted and plated at 1x104 per 24-well microplate wells for the experiments. The cells were allowed to adhere for 24hours at 37oC+5% CO2 and formed a layer at the cells at the bottom of the well. The hydrogel extracts were applied on them after 24 hours. Three samples, namely pure bacterial cellulose, BC/P, bacterial cellulose + Jacaranda caroba dye, BC/JC, bacterial cellulose + Calendula officinalis tincture, BC/CO were used for each hydrogel. The extract of the hydrogels was changed every two days. The culture medium was aspirated at each time point for the AlamarBlue® assay, and 500μL of 10% v/v% AlamarBlue solution were dissolved in DMEM culture medium (supplemented as described above) per well. The plates were maintained at 37°C+5% CO2 for 4 hours, and then 100 μL of the supernatant from each well were transferred to a 96-well microplate. 100 μL of completely reduced AlamarBlue® solution were also added to four wells of the new microplate for the calculation of the relative reduction percentage. The microplate was obtained through the autoclaving of the solution for 15minutes, according to the manufacturer's guidelines.17 The fluorescence values were measured by a fluorimeter (SpectraMax Gemini XS - Molecular Devices) using 544 nm excitation and 590nm emission spectrums. The values obtained from each sample were compared to the average of the fluorescence values obtained from the completely reduced AlamarBlue® solution. The assay was performed at periods of 3, 7, 14 and 21 days after the start of cell culture and data were analyzed by GraphPad Prism (GraphPad Software, La Jolla California USA) at a 5% significance level and two-way ANOVA with Tukey post-test.
Animal experimentation
The experiment was approved by the Ethics Committee on Animal Experimentation at the Faculty of Pharmaceutical Sciences at Araraquara, UNESP, São Paulo, Brazil. Thirty-two adult male rats (Rattus norvegicus, albinus, Holtzman) weighing approximately 250 g were used and randomly allocated into four groups, namely BC/JC hydrogel (GI), blood clot (negative control) (GII), BC/CO hydrogel (GIII) and pure (P) BC hydrogel (positive control) (GIV). Eight animals were studied per group/period. The periods for macroscopic and histological analyses were 3, 7, 15 and 30 days after surgery. General anesthesia was induced by intramuscular injections of ketamine hydrochloride (25mg kg-1; Agener União, Brazil) and xylazine hydrochloride (5mg kg-1; Bayer, Brazil). All surgical procedures followed standard aseptic protocols. After the shaving and preparation of the dorsal region, two wounds were made (2cm distance between them) in its medium line by a circular 10mm diameter scalpel. The depth of the wound reached epidermis, dermis, hypodermis and muscular layers until fascia superficialis. Each animal received two groups. For the first analysis, BC/JC hydrogel wound dressing (GI) was applied in the superior wound and the inferior wound was filled with a blood clot (GII), whereas for the second analysis, BC/CO hydrogel wound dressing (GIII) was applied in the superior wound and the inferior wound received pure BC hydrogel (GIV). The wound dressings were applied once per day during the whole treatment period. The animals were transferred to separate cages properly isolated and identified and kept at controlled room temperature (22±2°C), humidity (60-70%) and light (12 h light/dark cycles) under appropriate conditions of food, water and hygiene. Their recovery was monitored daily. In the immediate postoperative period, all animals received an intra peritoneal administration of a single dose of 12.5mg kg-1 tramadol analgesic (Medley Pharmaceutical Industry Ltda., Brazil).After 3, 7, 15 and 30 days, four animals in each group were anesthetized again, according to the protocol, for macroscopic analysis and removal of wounds. Subsequently, they were euthanized with an intramuscular administration of Thiopentax (thiopental, 0.16mL 100g-1 body weight; Cristália).
Macroscopic analysis
The wounds were macroscopically evaluated 3, 7, 15 and 30 days after surgery for analyses of the redness in the wound region, presence of excessive exudate and humidity. The lesions were photographed after the pre-euthanasia anesthesia procedure by a Sony digital camera (16.2-megapixel Cyber-Shot model), with the defined scale of all animals in each period. ImageJ software was used for the analysis of the area of lesions, whose measures were tabulated and statistically evaluated by GraphPad Prism software (GraphPad Software, La Jolla California USA). The level of statistical significance was established at 5%.The quantitative data obtained were tested for normality by Lilliefors Test. Two-way ANOVA followed by post-hoc Tukey were then applied for the evaluation of differences among the groups and effect of the follow-up period.
Histological analysis
After euthanasia, the areas of the wounds were surgically removed, fixed in a Bouin solution for 48h and processed according to the histological routine for light microscopy. The specimens were cut into semi-serial 6 µm sections in the longitudinal direction of the skin and stained with hematoxylin-eosin (H&E). They were analyzed and photographed under a microscope (Olympus X51; Olympus, WA) coupled to a digital camera (Olympus DP71, 12.5 Mpixels; Olympus) of 6.3× magnification. The images were captured by Leica Application Suite software. Scores of inflammatory reaction evaluations were given according to the inflammation degree (scores 0–4: 0 – no reaction; 1 - very slight reaction; 2 – mild reaction; 3 – moderate reaction; 4 – marked reaction) and ASTM F981–04 standards.18 The statistical analysis was descriptive and consisted in the division of the samples of each lamina (six laminas of each specimen with six cuts) according to 3, 7 and 15 – day periods. The first and last cuts were discarded. The tissue repair pattern was also evaluated regarding quality, quantity and orientation of collagen fibers and epithelium regeneration. The scores used are shown in Tables 1 & 2. GraphPad Prism software (GraphPad Software, La Jolla California USA) was used for the statistical analysis and the level of statistical significance was established at 5%. The data obtained were tested for normality by Lilliefors Test. ANOVA test was used for the analysis of inflammation degree and t-test was applied as a post-test. Kruskal-Wallis and Dunn tests analyzed the tissue repair standard (quality, quantity and orientation of collagen fibers) and tissue repair standard (epithelium reconstitution), respectively.
Classification |
Score |
Absence of fibers |
0 |
Disorganized fibers |
1 |
Poorly organized fibers |
2 |
Medium organization of fibers |
3 |
Well organized fibers |
4 |
Table 1 Classification of the tissue repair pattern regarding quality, quantity and orientation of collagen fibers
Classification |
Score |
Unrepaired |
0 |
Healing process |
1 |
Repaired |
2 |
Table 2 Classification of the tissue repair pattern in relation to the epithelial repair
Methanolic extracts of C. officinalis flowers and J. caroba leaves were characterized by HPLC. The chromatographic profile obtained for tinctures and extracts under standard conditions, called "digital printing", revealed their qualitative constitution and characterized the material analyzed. The chromatographic profiles of methanolic extracts of C. officinalis flowers and J. caroba leaves showed the presence of substances with a predominance of peaks ranging from 17 to 32 min and 13 to 26 min, respectively. No peaks of significant intensities were observed after this period. The profile of the methanolic extract of C. officinalis flowers revealed a predominance of 8 peaks (Figure 1A). The chromatogram showed peaks of 31.40 min retention time and ultraviolet spectrum of 254nm maximum wavelength, thus demonstrating the main substances present in the methanolic extracts of the C. officinalis leaves belong to this class. A phytochemical study of the extract of Calendula officinalis detected the presence of flavonoids, carotenoids and terpenoids. According to Copp19 terpenoids are the secondary metabolites considered the main promising class of anti mycobacterial activity. Studies conducted by Higuchi20 revealed the main terpenoids isolated that showed an inhibitory activity of Mycobacterium growth were lupeol, ursolic and oleanolic acids. Their presence in the methanolic extracts of flowers suggests they are also responsible for an anti mycobacterial action. The results corroborated the popular use of C. officinalis for the treatment of infections caused by microorganisms and revealed substances of the terpenoid class are responsible for the antimicrobial action.
A phytochemical study of the extract of J. caroba detected the presence of phytochinoids, triterpenes, phenols and flavonoids. The profile of the methanolic extract of those leaves showed predominance of 10 peaks (Figure 1B). The chromatogram revealed peaks of 18.95min retention time and ultraviolet spectrum of 254 nm maximum wavelength, thus demonstrating the main substances present in the methanolic extracts of J. caroba leaves belong to this class. According to Simões19 some flavonoids are responsible for an antitumor activity and may also act as antiviral, antibacterial, antihemorrhagic, hormonal, vasodilator, anti-inflammatory, antimicrobial and antioxidant. Carotenoids exert a favorable effect on the epithelization process, influencing the cell cycle progression of fibroblasts.21,22 The content of compounds, as flavonoids, carotenoids, terpenoids, triterpenes and phenols in the present study was similar to those reported in the literature. External factors, such as temperature, wind, soil, latitude and altitude, and technical factors, as planting, fertilization, harvesting season and growth stage can influence the chemical composition of the plants interfering with the content of the active principle and production of biomass.23
Physical-chemical characterization
Scanning electron microscopy (SEM): Figure 2 shows SEM images of lyophilized pure BC, BC/JC and BC/CO hydrogels, respectively. The porosity of the hydrogels is an important factor in the modulation of cell and tissue interactions and penetration of cells into scaffold.24 The images display a porous material, typical of a hydrogel, and the SEM micrographs show a structure of randomly disposed fiber arrangements, with some more compact points. Interconnected pores in three-dimensional directions throughout the samples are fundamental for the tissue repair, since such a morphology enables the in growth of fibroblasts.25 According to Figure 2, the surface of (a) apparently shows more compact fibers than (b) and (c) and the pores in the cross-section of (A) and (B) seem larger than those in (C). Distributed and interconnected fibers in layers are more visible in (A). Previous studies have shown hydrogels with large pores promote a better cell penetration and formation of new tissues within the microstructure of the scaffold, in comparison to hydrogels of lower porosity.24
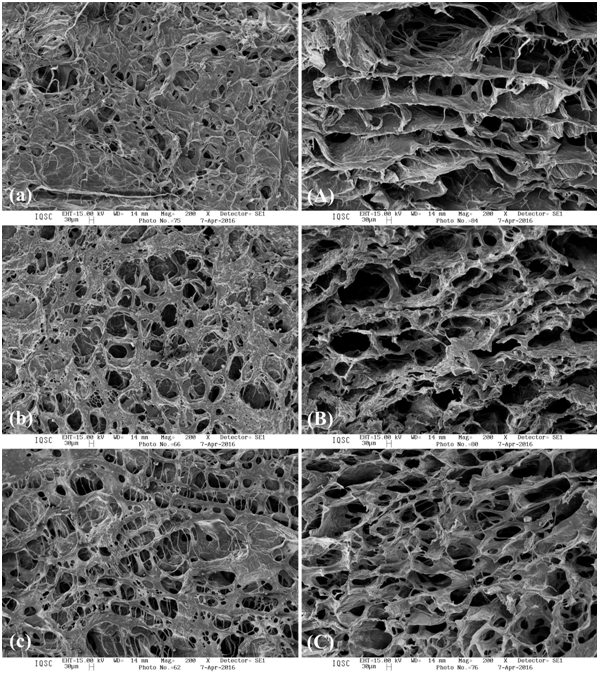
Figure 2 SEM surface images of BC/P (A), BC/JC (B) and BC/CO (C). SEM cross-section images (A, B and C).
Thermogravimetric analysis (TG): The samples were heated at a constant temperature until their mass had changed. At this point, the temperature stopped increasing, so that the mass could stabilize. The temperature increased again until the next mass change. Figure 3 shows the thermogravimetric curves obtained at 10 °C/min heating rate for the hydrogels. The three stages observed for the samples are attributed to:
The thermogravimetric waves of BC/P, BC/JC and BC/CO hydrogel samples showed in stage II, where the largest mass loss occurs, were achieved at 76.72 °C, 80.75 °C and 88.11 °C temperatures, respectively. BC/CO hydrogel showed a better result compared to BC/P and BC/JC hydrogels, since it supported temperatures of 13% and 8.3%, respectively, for its decomposition. However, BC/JC is better than BC/P (positive control) because it has a temperature of 13% more for its total decomposition.
Fourier transform infrared (FT-IR): Infrared radiation (IR) corresponds to part of the electromagnetic spectrum between the visible and microwave regions. Each functional group absorbs a given characteristic frequency, which enables the IR to characterize the functional groups of a standard or an unknown material26 through a graph of intensity of radiation versus frequency. The FT-IR technique is not destructive and accepts sparingly soluble sample spectra in solid state films, as lyophilized hydrogels.27 According to Figure 4, which shows similar infrared spectra among the lyophilized samples of the hydrogels, the chemical composition of BC did not change, i.e., its properties were maintained even after the addition of the dyes. The samples exhibited typical bands for proteins at approximately 1656 and 1547cm-1, related to the C=O amide I stretch and N-H deformation for amide II, respectively. These bands were observed with little intensity difference between the sample spectra, but the BC/JC hydrogel was slightly larger. The C-H band of aromatic is attributed to the band around 2922cm-1. The spectra showed bands around 1600-1700 cm-1, characteristic of a protein, which indicates the stretching of a carbonyl; at 1450cm-1, it indicates the presence of stretches of the phenyl ring (C=C). The absorption band at 1048cm-1 is characterized by the C-H bond of the ring. Despite some slight variations in the intensity of the detected vibrational modes, the values obtained are in agreement with those reported in the literature.28 The increase in the bands observed in the three samples may be related to the formation of hydrogen bonds between the hydroxyls of bacterial cellulose.29
Cytotoxicity and cell viability: L929 cells proliferated with the hydrogels extracts of pure bacterial cellulose (BC/P), bacterial cellulose with Calendula officinalis (BC/CO) dye and bacterial cellulose with Jacaranda caroba dye (BC/JC) uniformly up to 14 days after plating. The results show the final concentration of Calendula extract, estimated at 0.7m/v%, is not toxic to cells. The toxicity of repeated doses of extracts of Calendula officinalis flowers can be considered low, however, care must be taken in oral administration for prolonged periods, especially in individuals with liver, renal or hematological problems.30 The acute toxicity of the hydroalcoholic extract of Calendula officinalis (extracted with 30% ethanol, and with a plant extract: extractor liquid ratio of 1:1) administered subcutaneously was reported to be 45 mg in mice.30 Silva detected a low toxicity in the hydroalcoholic extract of C. officinalis flowers. They evaluated the oral administration of water extract at doses of 0.025; 0.25; 0.5; and 1.0 g/kg daily for 30 days in male Wistar rats and observed both hematological and biochemical parameters remained within normal limits.31 According to Brazil,30 an unpublished Cosmetic, Toiletry and Fragrance Association occlusion test involving aqueous extract of 10% C. officinalis performed in nine rabbits showed the primary index of dermal irritation was zero. Fronza et al. (2009) demonstrated the effects of the increase of C. officinalis ethanolic extract (10 g plant material/150 mL extractive solvent) fibroblast proliferation, at a 10 μg/mL concentration in the microplates, were 64.3 and 74.5% in relation to the control treatment (0.25% DMSO). According to Hernandes the extract of Caroba showed more cytotoxicity in comparison with other specimens of the Bignoniaceae family. However, it did not show signs of toxicity in our in vivo test, which agrees with the results of the macroscopic and histological analysis that revealed a better tissue regeneration rate (Figures 5 & 6) than the control (clot) for periods of 3 and 7 days, and relative to the BC/P hydrogel for the 3-day period. On day 21, the number of viable cells cultured with BC/JC (Figure 7) decreased as well as for the negative control (untreated), whereas for BC/P and BC/CO, the relative fluorescence remained stable. As the decrease also occurred for untreated cells, the decrease in the relative fluorescence for microplates with BC/JC hydrogel can be attributed to the cell dynamics. Under in vitro conditions, the cells towards filling all available space. The proliferation stabilizes and decreases due to the effect of the apoptosis signaling cascade. Apoptosis, i.e., the process of cell death, is fundamental for the development and function of multicellular organisms.34,35 Since the relative amount of viable cells remained stable for the other hydrogels (BC/P and BC/CO), aspects, as density and polymerization of such hydrogels should be investigated. If they are higher than those of BC/JC, a larger amount of bacterial cellulose is in contact with the cells, which increases the surface on which the cells can proliferate and maintains cell culture in vitro longer than the flat surface of the control well.36,37

Figure 5 30-day period, (A) – GI shows complete and information neovascularization, organized granulation tissue, mild inflammatory reaction in the complete epithelium, keratin layer, hair follicle and glands; (B) and (D) – GII and GIV show complete and information neovascularization, organized granulation tissue, medium inflammatory reaction in the complete epithelium and keratin layer; (C) – GIII shows complete and information neovascularization, organized granulation tissue, mild inflammatory reaction in the complete epithelium. Epithelium (A), crust (B), neovascularization (C), inflammatory infiltrate (D), granulation tissue (E) keratin layer (F), glands (G) and hair follicle (H). Staining: H&E.
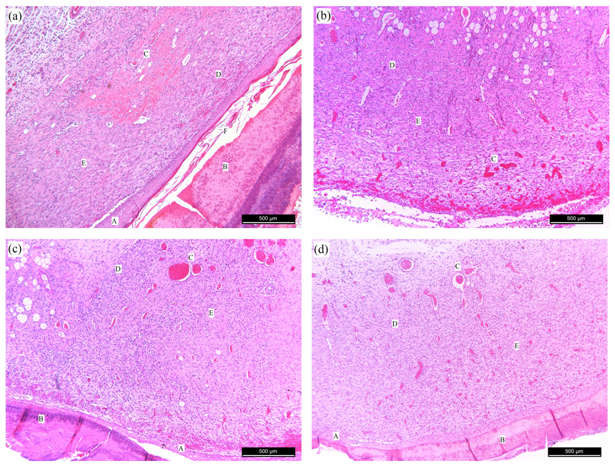
Figure 6 3-day period, (A) – GI shows complete and information neovascularization, crust, organized granulation tissue and moderate inflammatory reaction in the epithelial healing process; (B) – GII shows absence of epithelium, crust, disorganized granulation tissue, moderate inflammatory reaction and early neovascularization; (C) – GIII shows complete and information neovascularization, granulation tissue in organization and medium inflammatory reaction in the epithelial healing process; (D) – GIV shows complete and information neovascularization, crust, granulation tissue in organization and medium inflammatory reaction in the epithelial healing process. Epithelium (A), crust (B), neovascularization (C), inflammatory infiltrate (D), granulation tissue (E) and keratin layer (F). Staining:H&E.
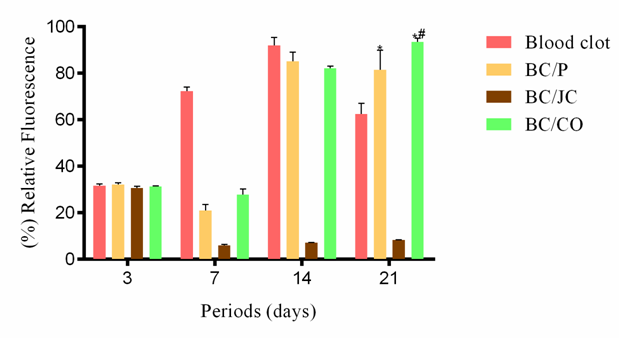
Figure 7 7-day period, (A) – GI shows complete and in formation neovascularization, crust, organized granulation tissue, keratin layer and moderate inflammatory reaction in the complete epithelium; (B) – GII shows absence of epithelium, complete and information neovascularization, organized granulation tissue, moderate inflammatory reaction; (C) and (D) – GIII and GIV show complete and information neovascularization, crust, organized granulation tissue and moderate inflammatory reaction in the epithelial healing process. Epithelium (A), crust (B), neovascularization (C), inflammatory infiltrate (D), granulation tissue (E) and keratin layer (F). Staining: H&E.
In vivo experiment
Studies of the wound healing process use, basically, three general parameters, namely wound histological analysis, including cell and extracellular matrix observation, quantification of the reepithelized area, and macroscopic wound qualification (which shows the presence of clot, secretions, hyperemia and hair).38 A large quantity of exudate and toxin is observed in wound contaminations and may retard the healing process and cause systemic infections.39 Different wound dressings have been developed due to the existence of several types of acute and chronic wounds caused by multiple pathophysiologies, which affect the soft tissues in different chronicity levels and wound healing phases. Alternative methods of wound closure have been designed and refined over the past decades.40 An ideal wound dressing should prevent exacerbated healing, promote no foreign body reaction, and be easily removed, if necessary, with no damage to the newly formed tissue.41 Healing depends on two pillars, namely vascularization and capacity of the cell to synthesize collagen. Vascularization promotes the transport of cells to the inflammatory site and supply of nutrients and oxygen. Furthermore, epithelial integrity is an important parameter for the definition of time and effectiveness of second-intention healing.42 Several factors can influence the stages of healing, and the main local ones include bleeding, oxygen tension and infection. Some extrinsic factors are surgical technique, topical antiseptics and dressings.43 Regarding wound dressings, several types based on plant herbs,44,45 hydrocolloid, alginate and collagen46 have been developed and can be applied as ointment, film,47 foam and gel48 for several treatments.49 This manuscript reports on the development of a wound dressing for tissue repair based on bacterial cellulose with C. officinalis and J. caroba.
Hydrogels promote suitable physiological conditions for wound healing50 and a barrier against microorganisms and enable the permeation of only water and nutrients. They also retain water; therefore, a favorable environment for wounds must be maintained for tissue repair. Epidermal cells can migrate at a 0.5mm/day rate along a moist wound surface, which is twice faster than a crust on dry wounds.51 Hydrogels have been indicated for the treatment of deep and contaminated wounds with high amounts of exudates.52 Their soft and rubbery feature minimizes irritation in adjacent tissue.53 Some positive points for the topical covers are: is not necessary to make sutures, they have a quick application and do not invade surrounding tissues. These characteristics favor the obtaining of efficient results when hydrogel is used for wound repair.8 The characteristics of bacterial cellulose, as transparent wound dressing, easy application and removal can be advantageous in cases of exacerbated healing (fibrosis) and/or exudates.41 Bacterial cellulose has been successfully used as a dressing for chronic ulcers, burns, dermabrasion and skin donor areas.54 Barud37 developed a wound dressing based on BC/propolis that proved an effective material in infected wounds because of its antibacterial action. The use of propolis and extract of J. caroba leaves, which show a dark coloration, causes the dressing to lose transparency. Since C. officinalis extract is clearer because it originates from the white flowers of the plant, transparency is maintained. However, BC/CO and BC/JC showed efficient wound dressings and promoted a faster and better re-epithelialization within 7-15 days (critical periods) of postoperative period in relation to other groups. They also showed easy application and good adhesion to the wound bed and caused no complications during the healing period. BC/CO yielded a macroscopically better result of tissue repair in relation to other hydrogels and exhibited most desirable characteristics for an ‘ideal dressing’. It was also suitable for the cleaning of dry wounds and promoted auto lytic debridement. The hydrogels were permeable to metabolites, decreased the temperature of the wound bed, which led to a marked reduction in pain, and were not reactive with the biological tissue. Moreover, they left no residue on the wound bed and improved the re-epithelialization of wounds.
No group showed formation of purulent exudate on the wounds. Such exudate is a sign of exacerbated inflammatory response probably caused by infection on the site or failure of material due to its cytotoxicity. The non-formation of the purulent exudate proved the good response of the organism to the coatings and enabled a more appropriate cicatricial process. This result is probably due to the excellent properties of bacterial cellulose, together with the antibacterial, anti-inflammatory and antifungal characteristics of the extracts of the medicinal plants present in the hydrogels. The mean values ± standard error of the wound sizes in relation to the experimental periods and groups are shown in Figure 8. The values in percentage were based on a previous study conducted by Sanchez. At days 3 and 7 (critical period) (Figures 5 & 6), all wounds showed a decrease in size, however, BC/CO showed a statistically significant difference (p<0.05) in comparison to the other groups. The lesions remained clean and a granulation tissue was formed mainly in the margins. Some studies have shown the formation of a larger vascular network in granulation tissue contributes toward a faster and better healing process due to the greater influx of nutrients and efflux of metabolites from the area of the wound. At day 15 (Figure 9), the size of the wounds had decreased and a complete reepithelization was observed only in the 3 groups treated. A statistically significant difference was detected in the tissue repair among the groups (p<0.05). BC/CO and BC/JC showed no statistical difference, however, pure BC hydrogel revealed a statistically significant difference (p<0.05) in comparison to the negative control group. At day 30 (Figure 10), no statistical differences were observed among the groups. Since the results of tissue repair were parametric, the Two-way ANOVA test was applied and showed a statistical difference between the treatments (p=0.0001). Tukey post-test revealed the best treatment in each period.
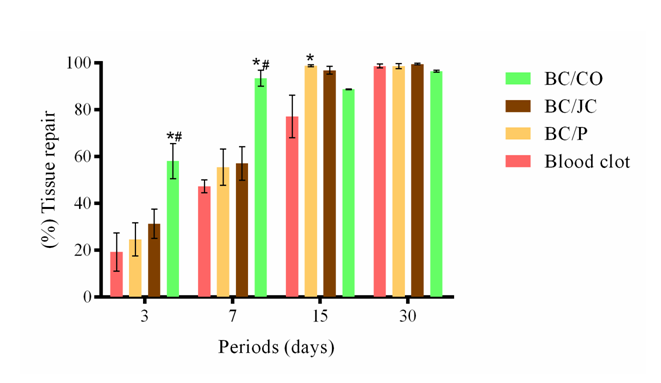
Figure 8 15-day period, (A) – GI shows complete and information neovascularization, organized granulation tissue, medium inflammatory reaction in the complete epithelium, keratin layer, dermis repair with hair follicle and glands; (B) - GII shows complete and information neovascularization, organized granulation tissue, crust, moderate inflammatory reaction in the complete epithelium; (C) – GIII shows complete and information neovascularization, organized granulation tissue, moderate inflammatory reaction in the complete epithelium, keratin layer, hair follicle and glands; (D) – GIV shows complete and information neovascularization, organized granulation tissue, medium inflammatory reaction in the complete epithelium, keratin layer and hair follicle. Epithelium (A), crust (B), neovascularization (C), inflammatory infiltrate (D), granulation tissue (E) keratin layer (F), glands (G) and hair follicle (H). Staining: H&E.
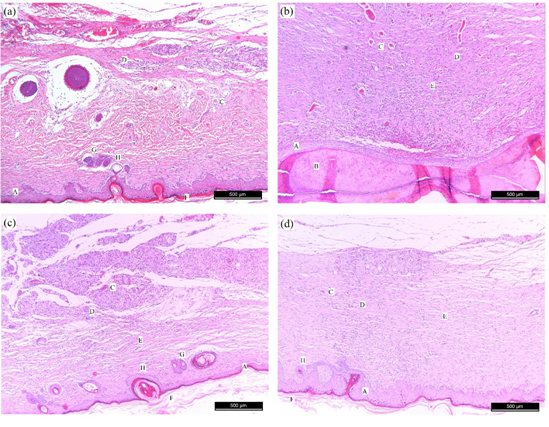
Figure 9 Values measured for the tissue repair of wounds of GI, GII, GIII and GIV groups for experimental periods of 3, 7, 15 and 30 days. Values are expressed as mean ± standard error; *p<0.05 in comparison to blood clot. #p<0,05 in comparison to BC/P.
A treatment with CB-CO promoted a statistical difference for tissue repair (p <0.05) in periods of three and seven days (critical periods), in comparison to clot-filled wounds (control) and CB-P. Observed the closure of wounds in a BC composite with chitosan and silver nanoparticles in a 25-day period. The toxic effects of silver on reepithelialization and cell proliferation must be taken into account61 and may justify a late closure. The dressings caused no exacerbation or prolongation of the inflammatory process because of their biological and physical properties and non-toxic, biocompatible and hydrophilic nature. An important aspect is their ability to contain exudate in the wound and form a capsule that immobilizes pathogenic microorganisms, which might cause an infectious process, hence, exacerbation and prolongation of inflammatory reactions. Although this study has revealed the test products exhibit no irritant properties on healthy skin even after repeated applications, some of their ingredients can cause skin sensitization after repeated uses over a longer period. Marigold is known to cause contact dermatitis after repeated applications due to sensitization. In a survey on the prevalence of sensitization to marigold in 443 consecutive patients, 2% reacted positively in the patch testing.62 In the current study, the hydrogels with the extracts showed no irritating properties probably due to their ideal concentration used for dressing preparations. The initial stage of healing, called inflammatory, is vital to the repair process. The role of the inflammatory phase, including the migration of neutrophils to tissues, has been debated in the literature, and most researchers believe neutrophils eliminate debris (cell fragments), protect the wound against infection, and promote healing. Neutrophil displacement begins immediately after a tissue injury and is short-lived, reaching a concentration peak in only 12 hours.
ANOVA was applied for the degree of inflammation and showed a statistical difference (p=0.037) between the treatments for the three-day period (critical period). T-test detected the group with best results and the treatment with the bacterial cellulose hydrogel with Calendula officinalis (BC/CO) showed a lower degree of inflammation (p=0.030) in relation to the hydrogel treatment of Pure Bacterial Cellulose (BC/P). Kruskal-Wallis test was applied for the degree of tissue repair standard (quality, quantity and orientation of collagen fibers) and showed a statistical difference (p=0.020) between the treatments for the seven-day period. Dunn post-test revealed the best group. The bacterial Cellulose hydrogel treatment with Calendula officinalis (BC/CO) achieved a better result than the treatments with Pure Bacterial Cellulose Hydrogel (BC/P) (p=0.0090) and Bacterial Cellulose Hydrogel with Jacaranda caroba (BC/JC) (p=0.014). Kruskal-Wallis test was applied for the degree of tissue repair pattern (reconstitution of the epithelium) and showed no significant statistical difference between the treatments.
BC/CO and BC/JC hydrogels are developed according to a practical and fast technique. They are biocompatible, of easy application and adhesiveness to the wound area, and their properties favor a the humid environment, faster tissue repair, less severe inflammation during critical periods in relation to other groups, and do not damage granulation. BC/CO maintained the semitransparent characteristic of CB after the addition of its dye. BC/JC showed a faster re-epithelialization (within 7 days) in comparison to the other treated groups, which exhibited this characteristic in the later period (15 days). SEM analysis revealed the presence of interconnected pores in the hydrogels, even after the addition of tinctures to BC. BC/P and BC/JC showed apparently larger pores, which favors the passage of cells for a faster re-epithelialization. TG analysis indicated the hydrogels with incorporated plant dyes displayed better thermal properties than BC/P. BC with dyes (10%) showed no toxicity to cell proliferation, which confirmed the concentration of dyes used, was favorable. BC/CO and BC/JC hydrogels are promising biomaterials for the tissue regeneration of complex wounds, mainly in the initial periods (chronic period) of healing, due to their favorable results in relation to the control groups (positive and negative).
The authors acknowledge the financial support provided by Brazilian agency CAPES.
Authors declare that there is no conflict of interest.

©2019 Moraes, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.