MOJ
eISSN: 2379-6294


Research Article Volume 5 Issue 1
1Department of Biological Sciences, Faculty of Science, Niger Delta University, Nigeria
2Department of Biology, Issac Jasper Boro College of Education, Nigeria
Correspondence: Sylvester Chibueze Izah, Ecotoxicology Research Unit, Department of Biological Sciences, Niger Delta University, Wilberforce Island Bayelsa State, Nigeria
Received: December 28, 2018 | Published: February 18, 2019
Citation: Izah SC, Enaregha EB, Epidi JO. Vitamin content of Saccharomyces cerevisiae biomass cultured in cassava wastewater. MOJ Toxicol. 2019;5(1):42?45. DOI: 10.15406/mojt.2019.05.00151
This study assessed vitamin content in Saccharomyces cerevisiae biomass cultured in cassava wastewater. The Saccharomyces cerevisiae was isolated from palm wine following microbiological techniques. The Saccharomyces cerevisiae was inoculated into the cassava wastewater and incubated at room temperature for 15 days. During the processes the medium was shaken intermittently between 7.00 to 19.00 hours’ daily. At the end of the experiment, the medium was decanted and then filtered using Whatman filter paper. The trapped biomass was washed with distilled water and then re-filtered. The resultant biomass in the filter paper was oven dried and analyzed for vitamins following standard protocol. The mean value of the vitamin were 40.73±7.94 mg/100g (vitamin D), 2280.37±105.85µg/100g (Vitamin A), 132.54±14.00µg/100g (Vitamin E), 6.88±1.62mg/100g (Vitamin C), 0.58±0.10mg/100g (Vitamin B12), 2.62±0.92mg/100g (Vitamin B3), 1.05±0.21 mg/100g (Vitamin B1). Based on the value obtained from the finding of this study, the biomass is a good source of vitamins that could be utilized in animal feed. Through utilization of the wastewater for Saccharomyces cerevisiae cultivation the attendant environmental impacts associated with the cassava wastewater could be minimized.
Keywords: animal feed, biotechnology, cassava wastewater, saccharomyces cerevisiae
Nigeria is the world leading producer of cassava accounting for over 20% of total global production.1–16 The production of cassava increased in Nigeria in the early 2000 due to several developmental projects that requires cassava as major raw material. Cassava cultivation and processing is a major source of livelihood to several families especially in the rural areas in southern Nigeria.15 Cassava is processed into garri, fufu, lafun Kigigha et al.,17 and used to feed livestock. It has several industrial applications as well.
In Nigeria over 60% of the total cassava tuber produced is used for the production of garri. Over 80% of garri production is carried out by smallholder that use rudimentary equipment for processing. During cassava tuber processing into garri large volume of wastewater is produced in the dewatering stage.12 This wastewater is often discharged into the ecosystem with little or no treatment.3–5 The wastewater often percolates in the soil, and may drain to a nearby pit or surface water. In aquatic ecosystem, the wastewater is injurious to fishes especially fingerlings.3,18 The wastewater also causes air pollution through the emission of offensive decomposing effluents.
Studies have shown that cassava wastewater has the tendency to alter soil microorganisms,15 soil heavy metals8–10,12 and general physicochemical characteristics. Previous studies have also suggested the need to manage the environmental impacts of cassava wastewater through bioethanol production16 and Saccharomyces cerevisiae biomass production for potential utilization in animal feed.5–7,13 To this effect, Saccharomyces cerevisiae biomass cultured in cassava wastewater have been studied with regard to amino acid,5 heavy metals,7 cations and cyanide.6 But information about the vitamin content of Saccharomyces cerevisiae is scanty in literature.
Typically, in living organisms, vitamins play essential role during metabolic processes. As such, the absence of certain vitamins could hinder metabolic processes leading to collapse of the actions. Vitamins are typically classified into two viz: water (vitamin C i.e. ascorbic acid, vitamin B1 i.e. thiamin, vitamin B2 i.e. riboflavin, Vitamin B3 i.e niacin, vitamin B12 i.e Cobalamin, vitamin B6, folic acid, pantothenic acid, and biotin) and lipid/fat (Vitamin A, D, E and K) soluble.19 The authors further reported that all water-soluble vitamins apart from Vitamin C have a catalytic function (that act as coenzymes of enzymes that is involved during the metabolism of carbohydrate, proteins and fats), and have specific functions in some specialized and differentiated tissues. On the other hand, lipid soluble vitamins aid in the maintenance of the integrity and structure of membranes, and they control the synthesis of some enzymes at the genetic level.19
Vitamin requirements tend to vary according to biological species and its genetic/biochemical variations as well as source of diets. This is because most of these vitamins are taken through vegetables including fruit of Synespalum dulcifcum (miracle fruit),20 Seed of Aframomum melegueta and Garcinia kola.21 Like human, livestock need minerals at certain amount for optimal productivity. This study aimed at investigating the vitamin content of Saccharomyces cerevisiae biomass cultured in cassava wastewater.
Sample collection
Untreated Cassava wastewater containing palm oil was obtained in replicate from a smallholder cassava processor in Ndemili, Ndokwa west Local Government Area of Delta state, Nigeria. The raw wastewater that contain palm oil was transported to the laboratory under ice pack using plastic container. The samples were used immediately at the laboratory.
Isolation and identification of saccharomyces cerevisiae
The Saccharomyces cerevisiae used in this study was isolated from palm wine bought from Rumoumasi, Port Harcourt, Nigeria. The pure isolate was made following the pour plate method by Pepper and Gerba,22 Benson23 using potato dextrose agar supplemented with chloramphenicol. The resultant pure isolate was identified following cultural, morphological, and physiological/biochemical characteristics (carbon fermentation and assimilation, glucose-peptone-yeast extract broth, lacto-phenol cotton blue stain and growth based on temperature) as previously described by Kurtzman and Fell,24 Benson23 and applied by Iwuagwu and Ugwuanyi,25 Abioye et al.,26 Okoduwa et al.27 The features of the isolates were compared with the guide provided by Ellis et al.,28 Kurtzman and Fell.24
Growth of the biomass and vitamin determination
The Saccharomyces cerevisiae biomass was grown following the method previously described by Izah et al.,3–7 Izah1 (Figure 1). The water soluble vitamins such as Vitamin C were analyzed with the spectrophotometric method previously described by Drevanasiddappa and Veena,29 while the other water soluble vitamins including thiamine (B1), Nicotinamide (B3) and Cyanocobalamin/cobalamin (B12) were analyzed based on the spectrophotometric method. Lipid and fat soluble vitamins such as Vitamin A, D and E was also analyzed following standard procedure.
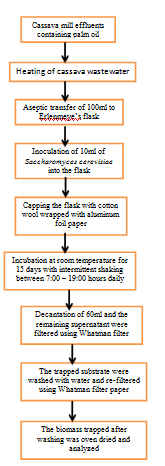
Figure 1 Schematics of Saccharomyces cerevisiae biomass production from cassava mill effluents (Modified from Izah et al., 2017a-e; Izah, 2018a, e).
Statistical analysis
SPSS software version 20 was used to carry out the statistical analysis. The results were expressed as mean ± standard deviation.
The vitamin content in Saccharomyces cerevisiae biomass cultured in cassava wastewater is presented in Table 1. The concentration of vitamin D was 40.73±7.94 mg/100g. Typically, vitamin D is essential for bone health probably due to the fact that it aids in the maintenance of serum calcium levels,30 proper mineralization of bone 19 as well as calcium and phosphorus ratio.31 Deficiency of vitamin D could lead to soft bone which could predispose the body to hunched back and rickets.
Vitamins |
Mean± Standard deviation |
vitamin A, µg/100g |
2280.37±105.85 |
vitamin D, mg/100g |
40.73±7.94 |
vitamin E, µg/100g |
132.54±14.00 |
vitamin C, mg/100g |
6.88±1.62 |
vitamin B12, mg/100g |
0.58±0.10 |
vitamin B3, mg/100g |
2.62±0.92 |
vitamin B1, mg/100g |
1.05±0.21 |
Table 1 Vitamin content in Saccharomyces cerevisiae biomass cultured in cassava mill effluents
The level of vitamin A was 2280.37±105.85 µg/100g. Vitamin A is a fat soluble vitamin which play essential role in many animals including bone formation,30,31 maintenance of epithelial cells and good vision.19,31 Vitamin A is also essential for reproduction, embryonic development, growth and immune response.19 Vitamin A deficiency in animals leads to morphological changes in bone,30 ocular disturbances that could lead to blindness and growth retardation.19,31 Typically vitamin A is found in cassava and have been reported in the range of 5.0- 35µg in cassava root32–36 and 8300 to 11800 µg in cassava leaves.35–37
In this study, the concentration of Vitamin E was 132.54±14.00 µg/100g. Like other vitamins, Vitamin E is important for fatty acid metabolism and maintenance of cell membrane.31 Vitamin E is among the lipid soluble vitamins that have antioxidant potentials,38 and has the tendency to interrupt free radical chain reactions and protect polyunsaturated fatty acids and cell membranes.19 Deficiency of Vitamin E could lead to muscle weakness,31 peripheral neuropathy and breakdown of red blood cells.19 The vitamin C concentration was 6.88±1.62 mg/100g. Vitamin C is a water soluble vitamin that is an essential cofactor for at least 8 enzymes.39 Some of the cofactors are essential for the hydroxylation of lysine and proline, thus it is important for the synthesis of collagen30 and dopamine.39 Vitamin C also has anti-oxidant and wound healing potentials,39 and could boost immune system and facilitate the absorption of non-heme iron (from plant foods).19 Deficiency of vitamin C could lead to slow wound healing processes, soreness and stiffness of joints, swollen and bleeding gums. Basically, vitamin C has been reported in the range of 14.9–50.0mg in cassava root32–36and 60 –370mg in cassava leaves.35–37
The level of Vitamin B12 in the Saccharomyces cerevisiae biomass was 0.58±0.10mg/100g. Typically, Vitamin B12 is essential for many physiological and metabolic responses including osteoblast function, iron metabolism,30 glucose production, metabolisation of methionine and cell growth.31 Baigent and Carpenter19 also reported that Vitamin B12 is an essential cofactor for enzymes for the metabolism of amino acids and fatty acids. The authors further reported that it is needed for the synthesis of new cells and neurological functions. Deficiency of Vitamin B12 could lead to gastrointestinal disturbances.19 The vitamin B3 concentration in the Saccharomyces cerevisiae biomass was 2.62±0.92 mg/100g. Vitamin B3 is a water soluble vitamin that is an essential component of coenzymes required for cellular metabolism, oxidation of fuel molecules, and synthesis of fatty acid and steroid disturbances.19 The authors further reported that deficiency of Vitamin B3 could lead to skin lesions and gastrointestinal disturbances. In addition, vitamin B3 is found in cassava and have been reported in the range of 0.6 –1.09 mg in cassava root32–36 and 1.3–2.8mg in cassava leaves.35–37
The level of vitamin B1 in the yeast biomass was 1.05±0.21 mg/100g. Like vitamin B3, vitamin B12 and Vitamin C, vitamin B1 is water soluble and is an essential component of a coenzyme involved in the metabolism of carbohydrate metabolism.19 Deficiency of Vitamin B1 could lead to nerves impairment.19 Typically vitamin B1 have been reported in the range of 0.03 – 0.88mg in cassava root.32–36 and 0.06 -0.31 in cassava leaves.35–37
Typically, Saccharomyces cerevisiae have been reported to play essential role preventing diarrhea and mortality, enhancing immune system, boosting of performance, milk production, fiber degradation and nutrient digestability, adsorption of toxic metal, stabilization of rumen pH and microorganisms in livestock’s.40 Based on the value of water soluble vitamins (B1, B3, B12 and C) and lipid soluble vitamins (A, D and E), introduction of Saccharomyces cerevisiae could also aid to improve the health status of livestock’s. In addition, most species of cassava are known to contain cyanide, but studies have indicated that heat treatment, additives such as palm oil and fermentation using Saccharomyces cerevisiae could significantly reduce to cyanide content.41,42
Vitamins are an essential ingredient in diet due to their roles in growth and metabolic processes in living organisms. Diet is the major source through which humans and livestock’s obtain necessary vitamins required for normal health. This study assessed the vitamin content in Saccharomyces cerevisiae biomass cultured in cassava wastewater and the result revealed the presence of water soluble (Vitamin C, Vitamin B2, Vitamin B3 and Vitamin B12) and lipid soluble vitamins (Vitamin A, D and E). As such, the cassava wastewater is a promising raw material for the cultivation of Saccharomyces cerevisiae biomass for possible utilization in animal feed industry.
None.
The author(s) declares that there is no conflict of interest.

©2019 Izah, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.