MOJ
eISSN: 2379-6294


Fermentation technology is as old as the history of man. Several foods are produced through fermentation technology. Fufu is produced from cassava through complete submerging of the cassava tuber in water to soften it, which is then sieved into slurry and dewater to some extent and cooked. This study investigated the variations in microbial density and in-situ water quality characteristics of cassava fermentation medium for fufu production. Triplicate samples of raw cassava tuber was obtained from a small-scale cassava processor in Ndemili, Delta state, Nigeria. The peeled cassava tuber was completely submerged in water for 0–144hours. At every 24hours interval the water from the fermentation medium was analyzed for microbial density and in-situ (pH, temperature, total dissolved solid, salinity, conductivity and turbidity) water quality. Results were in the range of 3.13-7.37Log cfu/ml for total fungi counts, 6.08Log cfu/ml for total heterotrophic bacteria counts, 115.80-4347.33mg/l for total dissolved solid, 165.90-10869.07µS/cm for conductivity, 65.50-4347.33ppm for salinity and 33.80-602.67NTU for turbidity, which increased as fermentation progressed. The pH decreased (tending toward acidity) as the fermentation period increased with a value range of 4.67-6.40. Temperature ranged from 28.67-29.20ºC. There was significant variation (p<0.05) between the various period of fermentation except for temperature. The findings of this study showed the variations that occurs during the fermentation of cassava tuber for fufu production.
Keywords: Fufu, In-situ water quality, microorganisms, fermentation dynamics
Cassava is among the major staple food in the world especially in many developing nations in Africa. Cassava is a major source of dietary carbohydrate. In addition, it is a starchy crop that stores its food in the root. Cassava is produced in many countries in the world. A significant amount of global cassava tuber is produced in Africa.1,2 Nigeria is the largest cassava producing nation in the world accounting for over 20% of global production.1–18 In Africa, cassava is also produced in Ghana, Congo Democratic Republic, Angola among others.1 Cassava is also produced in large scale in other countries of the world including Thailand, Indonesia, Brazil, Vietnam and India. In Nigeria an appreciable quantity of cassava tuber produced is used to produce gari (cassava flake). Izah2 estimated that about 60% of total cassava produced is used for the production of gari in Nigeria. The cassava cultivation and processing is dominated by smallholders that occupy about 80% of the sector. Like gari, flour, fufu are produced from cassava tuber. Cassava is also used as active ingredients for the production of livestock feeds, confectionaries, sweeteners, adhesives, some pharmaceuticals. In addition cassava is used for the production of bioethanol and cassava-bread. In many part of the Niger Delta, fufu processing is a major source of livelihood to some families. Like many other food, fufu is sold in markets, streets etc. Fufu is produced from fermented cassava slurry.18,19 The fermentation process involves soaking (complete submerging) of the peeled cassava tuber in water. The fermentation period varies ranging from 4–7days depending on the variety, age and other environmental condition (such as temperature) of the fermentation medium. Sometimes, the cassava tuber is washed and fermented with their peel which is then removed after the process.
Within 2 days of fermentation, bubbles begin to develop at the top of the fermentation medium, and during the process the cassava tuber begins to be soft. Instances of cassava tuber not been completely soft after 5days of fermentation have been observed. As such, some fufu processors occasionally used hot water for fermentation process so as to facilitate transformation of the cassava to slurry. Typically, fermentation process is as old as human history.20,21 During fermentation process several biological, physical and chemical changes takes place.22–24 Studies have also indicated that microorganism play essential role in the fermentation processes.20 To this effects several microbes including Staphylococcus aureus, Escherichia coli, Enterobacter, Pseudomonas, Bacillus, Micrococcus species (bacteria), Aspergillus flavus, Aspergillus niger, Penicillium, Rhizopus, Mucor, Fusarium, Geotrichum species (Fungi) have been isolated from maize fermentation medium for pap “ogi” production.23 Some of these microbes may be environmental contaminants that enter the medium through water and other materials used for the fermentation processes. Studies have shown the fermentation dynamics of in-situ and microbial density of maize22 and guinea corn.24,25 But information about the fermentation dynamics of cassava tuber used for fufu production is scanty in literature. Hence this study focused on in-situ water quality and microbial population of cassava fermentation medium for fufu production.
Field sampling
The cassava tuber used for the study was obtained from a small-scale cassava processor in Ndemili, Delta state, Nigeria. The cassava was peeled and transported to the laboratory in a jute bag and it was used after 12 hours of harvesting from the plantation.
Sample preparation
The cassava samples was washed with borehole water and then cut into pieces using knife and submerged completely in a 4 liter container. The cap of the container was loosely covered. Then after, 2ml of the fermentation water was obtained at 0, 24, 48, 72, 96, 120 and 144hours for the enumeration of microbial density. Furthermore, the in-situ characteristics (temperature, pH, conductivity, salinity and total dissolved solid) of the fermentation medium were determined by dipping the calibrated probe of the various water quality meters. Also 10ml of the fermentation water was collected for turbidity determination.
In-situ analysis
The in-situ characteristics analyzed in this study were carried out following manufactures guide. The pH was analyzed using pH meter (Extech DO700) following 3-point calibration (7.00pH, 4.00pH and 10.01pH). Total dissolved solid, conductivity, salinity and temperature of the medium was analyzed with a multipurpose meter (Extech EC400), while the turbidity was determined with turbidity meter (Extech Model TB400).
Enumeration of microbial density
Two media viz: Nutrient Agar (for total heterotrophic bacteria count) and Potato dextrose agar (for mould and yeast) were used in this study. Pour plate method previously described by Pepper and Gerba25 and Benson26 were adopted in this study. The water from the fermentation medium during the interval of study was serial diluted, and 1 ml was plated in sterile petri dish. Then after, prepared nutrient agar and potato dextrose agar was poured in the respective petri dish. The agar plate for total heterotrophic bacteria and total fungi was incubated for 24–48hours and 3-4 days respectively at room temperature. Then after, the colonies that grew on the various medium were counted and expressed as colony forming units (cfu)/ml of the cassava fermentation water.
Statistical analysis
SPSS software version 20 was used to carry out the statistical analysis of logarithm transformed microbial counts and in-situ water quality parameter. Data obtained from the triplicate investigation were expressed as mean±standard deviation. Significance level at α=0.05 was determined using one way analysis of variance. Waller-Duncan statistics was used to compare means. Pearson’s correlation matrix was used to show the relationship between the various in-situ parameters. The chart for Microbial density was plotted with Paleontological statistics software package by Hammer et al.27
The population of total fungi and total heterotrophic bacteria during cassava fermentation for fufu production is presented in Figure 1 & Figure 2 respectively. The microbial counts were 4.13Log cfu/ml at 0 hours, 5.86Log cfu/ml at 72hours and 7.37Log cfu/ml at 144hours for total fungi (Figure 1), and 6.08Log cfu/ml at 0 hours, 8.39Log cfu/ml at 72hours and 11.28Logcfu/ml at 144 hours for total heterotrophic bacteria counts (Figure 2). Significant variations (p<0.05) exist among the various time interval. Typically as the fermentation proceeds the microbial density increased. As fermentation proceeds the physical and chemical characteristics of the medium are altered. Kigigha et al.17 reported that as fermentation continues the diversity of microbes’ decreases and favour the proliferation of specific group of organisms. The trend of microbial density reported in this study is in consonance with the findings of Okowa et al.20 that reported an increase from 7.11Log cfu/ml at 0 hours to 11.17 Log cfu/ml at 96hours for total heterotrophic bacteria, and 4.16Log cfu/ml at 0 hours to 6.52 Log cfu/ml at 96 hours for yeasts/mould in maize fermentation medium. Kigigha and Kombo (2017) also reported that the density of total heterotrophic bacteria and total fungi increased from 4.62 Log cfu/ml at 0 hours to 6.85Logcfu/ml at 72hours and 3.78 Log cfu/ml at 0hours to 4.71Log cfu/ml at 72hours, respectively in a guinea corn fermentation medium. The variation could be due to difference in the biochemical characteristics of the substrates being fermented.
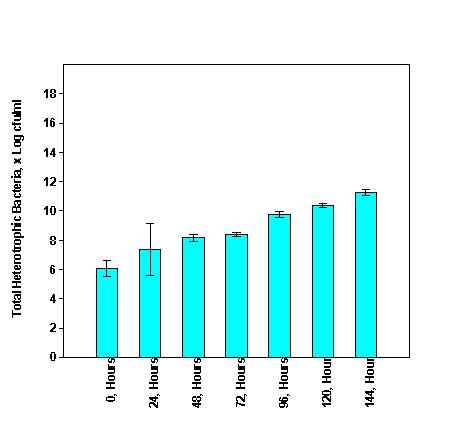
Figure 2 Population of total heterotrophic bacteria during cassava fermentation for fufu production.
The in-situ characteristics of cassava fermentation medium for fufu production between 0–144hours is presented in Table 1, while the Pearson’s correlation coefficient (r) matrix for the in-situ characteristics is presented in Table 2. The values were 165.90µS/cm at 0 hours, 2361.00µS/cm at 72hours and 10869.07µS/cm at 144hours for conductivity, 115.80mg/l at 0hours, 1652.40mg/l at 72hours and 4347.33mg/l at 144hours for total dissolved solid, 65.50 ppm at 0 hours, 944.30 ppm at 72 hours and 4347.33 ppm at 144 hours for salinity, 33.80NTU at 0 hours, 312.67 NTU at 72 hours and 602.67 NTU at 144 hours for turbidity, 6.40 at 0 hours, 5.46 at 72 hours and 4.67 at 144hours for pH. There was significant (p<0.05) increase in conductivity, salinity, total dissolved solid, turbidity and decline pH as the fermentation period increased. Temperature during the fermentation period (0– 144hours) ranged from 28.67-29.20ºC, being not significantly (p>0.05) different among the various period of fermentation. The increased in conductivity, salinity and total dissolved solid may be due the effect of the microbial consortia in the fermentation medium. Also the increase in these parameters also suggests that ions in the water have increased. As the fermentation medium continues the turbidity level increased due to microbial growth and changes in physical and chemical characteristics of the fermentation medium. This may have caused the medium to be become more acidic. Typically, cassava is acidic in nature and during its processing into gari, the effluents generated have been reported to be acidic.7,13 Conductivity showed positive significant relationship with salinity, total dissolved solid and turbidity and negatively correlate with pH at p<0.01. Total dissolved solid showed positive significant correlation with salinity and turbidity and negatively correlate with pH at p<0.01. Salinity positively correlates with turbidity and negatively correlate with pH at p<0.01, and turbidity negatively correlate with pH at p<0.01 (Table 2). The significant relationship between conductivity, salinity and total dissolved solid suggest that they mutually dependent.
Fermentation period, Hours |
Conductivity, µS/cm |
Total dissolved solid, mg/l |
Salinity, ppm |
Turbidity, NTU |
pH |
Temperature, ºC |
.00 |
165.90±5.57a |
115.80±3.40a |
65.50±1.97a |
33.80±6.58a |
6.40±0.02e |
28.77±0.65a |
24.00 |
704.03±4.43b |
491.93±2.74b |
280.97±1.25b |
87.63±5.03b |
6.19±0.02de |
28.70±0.44a |
48.00 |
1601.90±9.06c |
1121.43±7.00c |
640.77±4.48c |
181.93±9.45c |
5.99±0.03d |
29.20±0.30a |
72.00 |
2361.00±26.46d |
1652.40±18.28d |
944.30±10.44d |
312.67±11.57d |
5.46±0.13c |
28.90±0.78a |
96.00 |
3060.37±37.83e |
2141.77±27.08e |
1224.03±14.56e |
361.33±44.42e |
5.11±0.04b |
28.97±0.89a |
120.00 |
5322.07±91.08f |
2127.77±34.15e |
2127.77±34.15f |
525.13±26.00f |
4.87±0.15ab |
28.83±0.35a |
144.00 |
10869.07±391.00g |
4347.33±156.71f |
4347.33±156.71g |
602.67±26.50g |
4.67±0.25a |
28.67±0.32a |
Table 1 In-situ water quality parameter during cassava fermentation for fufu production
Each value is expressed as mean ± standard deviation (n=3); Different alphabets along the column indicate significant variation (P<0.05) according to Waller-Duncan Statistics
Parameters |
Conductivity |
TDS |
Salinity |
Turbidity |
pH |
Temperature |
Conductivity |
1 |
|
|
|
|
|
TDS |
0.968** |
1 |
|
|
|
|
Salinity |
1.000** |
0.968** |
1 |
|
|
|
Turbidity |
0.904** |
0.926** |
0.904** |
1 |
|
|
pH |
-0.847** |
-0.900** |
-0.847** |
-0.967** |
1 |
|
Temperature |
-0.140 |
-0.084 |
-.141 |
-0.033 |
0.103 |
1 |
Table 2 Pearson’s correlation matrix of the in-situ water quality parameter during cassava tuber fermentation for fufu production
**. Correlation is significant at the 0.01 level (2-tailed). N=21
The trend reported in this study has been reported in related studies. For instance, Kigigha and Kombo24 reported that salinity, conductivity, total dissolve solid and turbidity increased and pH decreased as guinea corn fermentation period increased. The authors reported a value of 99.10 ppm at 0 hours to 966.53 ppm at 72hours for salinity, 211.50 µS/cm at 0 hours to 1585.13 µS/cm at 72 hours for conductivity, 107.53 mg/l at 0 hours to 1320.22 mg/l at 72 hours for total dissolved solid and 59.63 NTU at 0 hours to 356.30 NTU at 72 hours for turbidity, and pH decreased from 6.40 at 0hours to 3.98 at 72hours. Okowa et al.22 also reported that as maize fermentation period increased the in-situ parameters increased from 114.67 ppm at 0 hours to 1663.33 ppm at 96 hours for salinity, 230.00µS/cm at 0 hours to 2903.33 µS/cm at 96 hours for conductivity, 161.33mg/l at 0 hours to 2076.73mg/l at 96 hours for total dissolved solid and 65.17NTU at 0 hours to 473.33NTU at 96hours for turbidity, and pH decreased from 6.37 at 0 hours to 3.83 hours at 96 hours, and temperature fluctuates between 28.47–29.57ºC. Again, the variation in the values could be due to the biochemical characteristics of the substrates.
Microorganisms play essential role during the fermentation of cassava tuber for fufu production. They are vital in determining the quality of a specific food. Throughout human history several food products have been produced through fermentation. Fufu is commonly produced and consumed in many areas in Nigeria. During fermentation of the cassava tuber for slurry preparation several physical, chemical and microbiological activities take place. This study investigated the changes in microbial density and in-situ water quality characteristics during fermentation of cassava tuber for fufu production. The study showed an increased microbial population (total heterotrophic bacteria and total fungi) and in-situ water quality (viz: salinity, conductivity, total dissolved and turbidity, and a decline in pH (tending toward acidity).
None.
The author(s) declares that there is no conflicts of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.