MOJ
eISSN: 2374-6920


Proceeding Volume 1 Issue 1
1Australian School of Advanced Medicine, Macquarie University, Australia
2David Geffen School of Medicine, Department of Biological Chemistry, University of California Los Angeles (UCLA), USA
3Department of Chemistry and Bimolecular Sciences, Macquarie University, Australia
Correspondence: Margaret Simonian, David Geffen School of Medicine, Department of Biological Chemistry, University of California Los Angeles (UCLA), 611 Charles E. Young Drive East, CA. 90095, USA, Tel +13107947308
Received: April 25, 2014 | Published: April 30, 2014
Citation: Simonian M, Ogorzalek Loo RR, Loo JA, et al. Proteomics detection of endothelial cell surface proteins following irradiation as potential targets for brain arteriovenous malformations molecular therapy. MOJ Proteomics Bioinform. 2014;1(1):4-7 DOI: 10.15406/mojpb.2014.01.00002
Arteriovenous malformations (AVMs) in the brain are vascular neurological lesions consist of abnormal collection of arteries and veins. They are the most common cause of brain haemorrhage in children and young adults. Over 90% of large AVMs (>3cm) are not curable, or curable with unacceptable risks. Radiosurgery is the treatment option for lesions (< 3cm) in diameter and located in eloquent areas where surgery can cause neurological defecit. However vascular occlusion post radiosurgery can takes up to 3year to complete while patients remain at risk of haemorrhage. This study aims to develop a molecular therapy for human brain AVMs. Specifically we aim to identify unique protein targets of AVMs that are up-regulated post irradiation. These proteins will then be used to develop a ligand-directed vascular treatment that promotes rapid thrombosis in AVM vessels post radiosurgery.
AVMs consist of abnormal collection of arteries and veins. They are the most common cause of haemorrhagic stroke in children and young adults. Radiosurgery is the treatment option recommended for lesions less than 3cm in diameter and located in eloquent areas where surgery can cause neurological deficits. This study aims to identify endothelial protein targets of AVMs that are differentially expressed compared to normal vessels post radiosurgery, to develop a ligand directed vascular treatment that promotes rapid thrombosis in AVM vessels. This is the first time that proteomics approach has been used in AVMs study (Figure 1).
• Assess membrane protein changes in the murine endothelial cell culture (bEnd.3) in response to radiation over time using proteomics analysis.
• Determine candidate proteins location with immunocytochemistry.
• Protein candidates will be used for a ligand-directed vascular targeting treatment to promote rapid thrombosis in AVM vessels post radiosurgery (Figure 2).
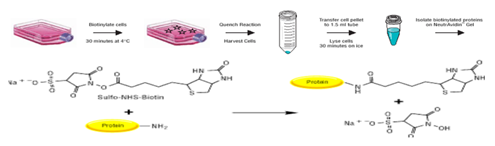
In vitro biotinylation and iTRAQ mass spectrometry was used to assess membrane protein changes in the murine endothelial cell cultures (bEnd.3). Cells were irradiated with 25Gy and surface biotinylation was performed using NHS-LC-Biotin at 6h, 24h, 48h and 72hours post radiation. Biotinylated proteins were captured on streptavidin resin, digested with trypsin, and then labelled with iTRAQ 8-plex reagents kit (Applied Biosystems). Peptides were separated by SCX and analysed by nanoLC/ MS on Qstar Elite (AB Sciex) (Figure 3).
Immunocytochemistry was used to validate candidate proteins location and expression in bEnd.3 cells.
In vivo biotinylation of the rat model of AVM was also conducted. The rat was perfused with NHS-LC-biotin, the fistula was harvested, membrane proteins were extracted and captured on streptavidin resin, digested with trypsin and analysed by LC/MS.
NanoLC ESI MS/MS data were submitted to Protein Pilot V4.0 (AB Sciex). iTRAQ MS identified on average 112 proteins from 1120 distinct peptides from 3 independent biological replicates. The detected protein threshold (unused ProtScore) was set as >1.3 (better than 95%confidence). 11 proteins were significantly differentially expressed in at least 2 out of 3 experiments. The most extensive changes were observed after 48h post radiation, e.g. cadherin 5 & CD109 (Table 1) (Figure 4‒8).1-12
Annexin A2 |
CD99 |
Biglycan |
ACO3 |
GP3 |
ATA1 |
Lumican |
PAT3 |
PTPRC |
CD36 |
AT1B1 |
Prolargin |
VCAM2 |
PECAM |
OME2 |
CAD13 |
Rab1B |
Annexin A1 |
Table 1 Mass spectrometry identified 120 proteins in the rat model of AVM, 32 of them were known as membrane proteins, e.g. in the table below
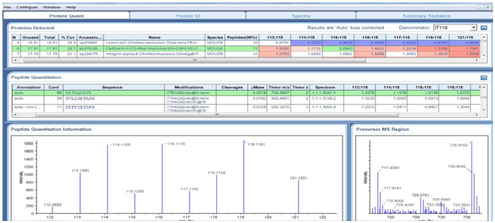

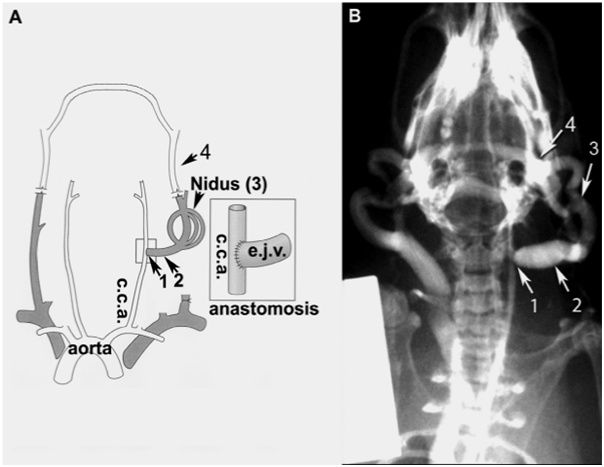
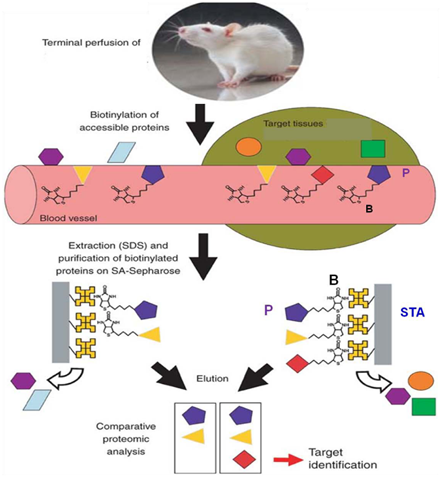
Cell surface protein biotinylation and mass spectrometry of murine endothelial cells identified protein targets in response to irradiation.
None.
The author declares no conflict of interest.

©2014 Simonian, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.