MOJ
eISSN: 2374-6920


Research Article Volume 4 Issue 4
Department of Biotechnology, Bapuji Institute of Engineering and Technology, India
Correspondence: Sreenivas Reddy Bathula, Department of Biotechnology, Bapuji Institute of Engineering and Technology, India, Tel +919591240234
Received: June 05, 2016 | Published: November 21, 2016
Citation: Bathula SR. MAGE (melanoma-associated antigen) protein purification and stability studies using CD spectroscopy and NMR. MOJ Proteomics Bioinform. 2016;4(4):261-267. DOI: 10.15406/mojpb.2016.04.00127
The SMC 5-6 protein complex is involved in the cellular response to DNA damage. It is composed of 6-8 polypeptides. The Nse1, Nse3 and Nse4 proteins form a tight sub-complex of the large SMC 5-6 protein complex. Human NSE3/MAGE-G1 the mammalian ortholog of Nse3 is the MAGE (melanoma-associated antigen) protein family founding member. The Nse4 kleisin subunit is related to the EID (E1A-like inhibitor of differentiation) family of proteins. We have recently shown that human MAGE proteins can interact with NSE4/EID proteins through their characteristic conserved hydrophobic pocket. Using CD spectroscopy and NMR we studied MAGE homology domain structure stability and its interaction with EID protein. Measurements of the MAGE-C2 binding affinity to EID-2 were done.
We developed purification protocols to get milligram quantities of different MAGE proteins and their fragments, express them in E. coli and purify them. The proteins were analyzed using NMR, studied protein-protein interaction of MAGE-C2-EID2 proteins using NMR techniques to test our hypothesis that the Nse3/MAGE proteins interact with common Nse4/EID binding and functional partners.
SMC complexes
The SMC (Structural Maintenance of Chromosomes) proteins play important roles in sister chromatid cohesion, chromosome condensation and DNA repair. Three separate SMC protein complexes are conserved in all eukaryotes. The cohesin complex (SMC1/3) is essential for cohesion of sister chromatids whereas condensin (SMC2/4) participates in chromosome condensation and segregation. The SMC5/6 complex is implicated in DNA repair and checkpoint responses.1–4
The SMC5/6 complex (Figure 1) from S. pombe is composed of four essential non-SMC components, designated Nse1-4.5 Reconstitution experiments with these components of the S. pombe complex identified two sub-complexes. SMC5, SMC6 and Nse2 form one of these, whereas Nse1, Nse3 and Nse4 form a second sub-complex. Nse4 bridges these two sub-complexes and functions as the kleisin subunit of the SMC5/6 complex.6,7 Analysis of the Nse2 subunit led to the discovery of its SUMO ligase activity in vitro and in vivo.8,9 Recently, it was suggested that the SMC5/6 complex may function as an E3 SUMO ligase complex specifically localized to damaged chromatin.10–12
MAGE proteins
The sequence of the Nse3 subunit is related to the MAGE protein family. The MAGE proteins were first described as cancer-testis antigens (MAGE-A, -B, -C; class I; Figure 2), which are silenced in normal adult tissues but aberrantly expressed in tumor cells.13 In tumor cells, some MAGE proteins are degraded, and the short peptides are a source of antigens that cause tumor rejection reactions when presented in the context of the major histocompatibility complex. Little is known about the processing of these cancer specific MAGE proteins. However, if the MAGE proteins are degraded through the most common ubiquitin/proteasome degradation pathways then specific ubiquitin ligases must bind them first.14
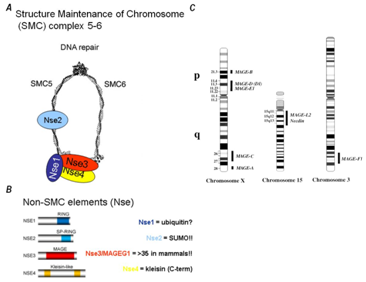
Figure 1 In this figure left panel (A), shows the architecture of SMC5/6 complex.3 There are two sub-complexes, the first comprising Smc5, Smc6 and Nse2, the second made up of Nse1, 3 and 4. Panel (B), Nse1 is an E3 ubiquitin ligase. Nse2 is a SUMO ligase. Nse3 is related to the MAGE family of proteins, which are expressed in many types of tumour cells. Nse4 is related to the EID family and functions as the kleisin. Panel (C), shows chromosomal localization of MAGE family genes. The MAGE-A to -E1 genes are located on chromosome X, while MAGE-L2, G1 and Necdin genes are located on 15q11-13. MAGE-F1 is on chromosome 3.4

Figure 2 Domain structures of selected MAGE family members are shown, with the MAGE homology domain (MHD) indicated in yellow and MHD2 in green. The jagged lines in the MHDs in MAGE-E1 and MAGE-H1 indicate the presence of truncated MHDs.12
Recently, a new class of MAGE genes was discovered (MAGE-D, -E, -F, G; class II). These genes are normally expressed in embryonic and adult tissues, especially the brain.15 The best characterized is the MAGE-D1 protein which functions as an adaptor protein that mediates multiple signaling pathways. The level of MAGE-D1 protein is tightly regulated through its interactions with the RING finger E3 ubiquitin ligases, Praja1 and Neurodap-1.16
The MAGE-G1 protein is the closest Nse3 orthologue and is present in the human SMC5/6 complex.17 As in yeast SMC5/6 complex, the human MAGE-G1 interacts with Nse1 and Nse4 subunits.
MAGE domain constructs and tags
MAGE domains of various MAGE family members were expressed using different vector constructs and tags.18 The best expressed was MAGE-A4 fragment (from aa 108 to 295) in pTriEx-4 vector (Novagen, USA). The pTriEx-4 has HIS-tag and S-tag for purification, thrombin and enterokinase enzyme cleavage sites for tag cleavage (Figure 3). The total number of amino acids in this construct is 236 and the calculated molecular weight is 26501.1191 Daltons.
MAGE-A4 fragment
MALSNKVDELAHFLLRKYRAKELVTKAEMLERVIKNYKRCFPVIFGKASESLKMIFGIDVKEVDPASN
TYTLVTCLGLSYDGLLGNNQIFPKTGLLIIVLGTIAMEGDSASEEEIWEELGVMGVYDGREHTVYGEP
RKLLTQDWVQENYLEYRQVPGSNPARYEFLWGPRALAETSYVKVLEHVVRVN.
As NMR spectra complexity increases with protein size, a long tag is not suitable forNMR structural studies. To get rid of any tags, we modified the vector and derived three variants.
pTriEx-4 without tag.
pTriEx-4 only with His-tag
pET-28 only with His-tag.
Another possibility to make the protein smaller and more feasible for NMR studies is to make the protein shorter by dividing it into two parts. Structural predictions suggest that the MAGE homology domain consists of two winged helix motifs. So we cloned each of the MAGE-
A4 MAGE domain motifs into pTriEx-4 and tried to express them. We were not successful in obtaining enough protein in soluble form.
Plasmid (pTriEx-4) transformation and isotope labeling
For 100μl of E.coli C41 (DE3) cells, 10μl of pTriEx-4 MAGE constructs were added and kept on ice for 20minutes, followed by heat shock for 40seconds at 42°C. The cells were then diluted with 400μl of LB media and grown for 1hour at 37°C. Then cells were transferred onto Petri dish containing ampicillin (100μg/ml). The Petri plates were incubated at 37°C overnight and the efficiency calculated based on the average number of colonies per plate.
From transformed E.coli C41(DE3) cells, one loop full of culture was inoculated into 3ml of LB medium with ampicillin. The culture was grown at 37°C (200rpm shaker) overnight. 3ml of overnight culture was transferred into 300ml of LB medium with ampicillin & cells were grown at 37°C (200rpm shaker). Pelleted cells were resuspended in fresh cold M9 media (2liters).
The growth of the culture was monitored by measuring optical density (OD) at 600nm. If there was an increase in OD (reached from 0.2 to 0.8) then cells are recovering. The flask was moved to 22°C and expression was induced with 1ml of 1M IPTG per liter of culture. The pellet was collected after 16hours and frozen at -70°C.
M9 salts minimal media
M9 salts are the minimal media used for unlabeled and labelled growths of E. coli strains. For small-sized peptides, NMR (e.g. backbone, side-chain, and NOE assignments) can be performed using the natural C13 and N15 abundance. Bigger proteins have to be labelled. Such labelled proteins are usually produced from cultures growing in (C13 glucose and N15 ammonium chloride) isotope labelled media.
The recipe of the M9minimal medium optimized for MAGE proteins expression is given in Appendix 2.7.1. Isotope labelled MAGE protein samples were prepared only for NMR experiments. Unlabeled MAGE protein samples were used for the CD spectra and for other experiments.
Cells were suspended in lysis buffer in a ratio of 1g cell wet weight to 1ml lysis buffer, then sonicate. Sonication is the most popular technique for lysing small quantities of cells (1-6 L of cell culture). Cells are lysed by liquid shear and cavitation. DNA is also sheared during sonication, so it is not necessary to add DNase to the cell suspension. The temperature is controlled by keeping the suspension on ice and using a number of short pulses (5-10sec) for 7minutes with 10mW power and 80 cycles (Ultrasonic processor (Sonics Inc.)). The cell debris was removed by ultracentrifugation at 4°C for 30min at 45 000rpm using a 45Ti rotor (Beckman). The supernatant was collected and used in His-tag affinity based purification.
Protein purification
Two different purification strategies were followed for both tagged and untagged protein purification. All the methods were according to the GE Healthcare protein purification handbook. A more detailed description of the purification methods is given in Appendix 2.7.2.
Purification methods followed for both tagged and untagged protein
Purified MAGE protein samples were used in NMR and CD spectra methods.
Circular Dichroism
Circular dichroism spectra were recorded at room temperature (22°C) using a Jasco J-810 spectrometer (Jasco, Japan). Data was collected from 185 to 260nm, at 100nm/min, 1s response time and 2nm bandwidth using a 0.1cm quartz cuvette containing the protein in 10mM phosphate buffer (pH 7.34). Each spectrum shown was the average of ten individual scans and was corrected for absorbance caused by the buffer. Collected CD data were expressed in terms of the mean residue ellipticity (ΘMRE) using the equation:
Where Θobs is the observed ellipticity in degrees, mW is the protein molecular weight, n is the number of residues, l is the cell path length, c is the protein concentration and the factor 100 originates from the conversion of the molecular weight to mg/dmol. Secondary structure content was calculated from the measured spectra by using CDSSTR,19–21 Selcon3,22–24 K2D25 and CONTIN26,27 methods implemented in DichroWeb server.28–30
Thermal unfolding of tested proteins was followed by monitoring the ellipticity at 222nm over a temperature range of 20 to 80°C, with a resolution of 0.1°C, at a heating rate of 1°C/min. Recorded thermal denaturation curves were roughly normalized to represent signal changes between approximately 1 and 0 and fitted to sigmoidal curves using Origin 6.1 software (Origin Lab, Massachusetts, USA). The melting temperatures (Tm)were evaluated as the midpoint of the normalized thermal transition.
NMR
The 1H-15N HSQC (Hetero nuclear Single Quantum Coherence) experiment correlates chemical shifts of directly bound NH group nuclei.
Using 2liters of 15N isotopically labeled media, we produced 10mg/ml MAGE-A4 protein (AA from 108 to 295, ~26.5kDa). It was used to prepare 700μl of 0.539mM protein sample in 25mM PBS (136-150mM NaCl), pH 7.34. The 1H-15N HSQC spectra were acquired at 25°C on 600MHz NMR Bruker Avance Spectrometer (equipped with TCI cryo probe head). The spectrum was acquired as a (1024x2) x (64x2) data matrix with spectral widths of 8389 and 2127 Hz for 1H and 15N.
The 1H-15N HSQC spectra were processed and analyzed using NMR Pipe31,32 and Sparky software (T.D. Goddard and D.G. Kneller, UCSF, San Francisco, CA, USA).
His trap purification
Generally, Nse3 (MAGE) protein expression levels, solubility and stability are low. It is hard to express them in large amounts to perform structural studies. I expressed various homologues and was successful in getting some pure protein samples. MAGE A3, A4, and C2 were over-expressed, and stability tests were done on these candidates for further studies. Purification results are shown only for the best of them i.e. MAGEA4. Samples were used in CD spectra and NMR methods to reveal structural data.
During protein purification we employed HIS-trap affinity based purification as the first step. The crude extract contains bacterial proteins and MAGE-A4 protein (Figure 4). It is an important step to remove other proteins, and capture His-tagged MAGE-A4 protein.

Figure 4 His-Trap affinity column used to purify histidine-tagged MAGE-A4 from bacterial extracts. Chromatogram shows the elution profile with red arrow representing purified fraction peak. The peak fraction samples were run a 15 % SDS/PAGE gel (top right window).

Figure 5 Mono Q column used for MAGE-A4 anion exchange based protein purification. Red arrow points to monomeric MAGE-A4 peak, green and black arrows point to contaminated and degraded protein. These fractions were analyzed on a 15 % SDS-PAGE gel (top right panel).
I used a linear imidazole gradient to determine the elution profile and a step gradient for actual elution. Longer washing of column with 37.5mM imidazole was used to reduce non specific binding. Even though I obtained a monomeric sample, it soon started to visibly aggregate/associate at room temperature. To minimise such aggregation, the sample was kept at low temperatures (fridge) and was used fresh for each study. For the elution of the target MAGE-A4 protein the column was washed with 300mM imidazole. We achieved yields close to 10mg/liter LB media.
Mono Q purification
Mono-Q Ion Exchange (IEX) was used as a part of the multistep purification. The estimated MAGE-A4 isoelectric point (pI) is 5.64, and charge at pH 7.34 is -3.9. An anion exchanger was used to separate target protein and its interacting proteins. The unbound molecules elute before the gradient begins and the tightly bound molecules elute in the high salt wash.
Inside this Mono-Q, monomers elute at low concentrations of salt compared to contaminated and degraded protein. In our observation the monomer eluted in between 50-70mM (Figure 5). The other fractions eluted in between 100-2000mM NaCl.
Gel filtration
Some members of the MAGE family proteins contain more than one MAGE domain (Figure 2). From previous studies34 we know that MAGE proteins can make dimers. We wanted to find the percentage of dimers in our protein samples. We used Sephadex-75 column for gel filtration. This column can be applied for a molecular weight range of 3-70kDa (globular proteins). Figure 6 shows that the majority of the MAGE-A4 protein was in monomeric form and 10% eluted as dimer. Due to aggregation/association of MAGE-A4 protein after every purification large protein loses were observed. His-Trap, ion exchange and gel filtration methods provided ~ 95% pure samples of MAGE-A4 MAGE domain (Figure 11).
From my ion exchange (Mono-Q) and gel filtration observations, I have seen dimers forming (Figure 6). Even though the MAGE domain forms a dimer in vitro, there is no in vivo evidence of such dimerization.
Cleavage of tag and concentrating MAGE-A4 protein
While concentrating MAGE-A4 protein by using Millipore Amicon Centrifugal Filter Units with 10kDa cut-off, I observed protein breakage at the end of the tag near the thrombin cleavage site (Figure 7). The tag gets broken easily - most likely because of mechanical forces. Enterokinase tag removal was performed, but the reaction never achieved 100% efficiently (Figure 8).

Figure 6 Separation of monomer and dimers using gel filtration. Red arrow points to monomer, black arrow points to dimer and a green arrow points to degraded protein. These fractions were analyzed on a 15 % SDS-PAGE gel (top right panel).

Figure 7 Concentrating MAGE-A4 protein using centricon. Samples were analyzed using mass spectrometry (MS). Top panel, normal N15 isotope labelled sample (MW = 26854 Daltons); bottom panel, centricon treated sample (MW = 22824 Daltons).

Figure 8 The 48 amino acid tag was cleaved using enterokinase. To get rid of the whole tag, centricon concentrated sample was treated with enterokinase. Samples were analyzed using mass spectrometry (MS). Bottom green panel shows centricon concentrated sample and top red panel shows enterokinase treated sample. These fractions were analyzed on a 15% SDS-PAGE gel (top right corner right to left) Samples with tag (~26 kDa), sample concentrated with centricon (~23 kDa) and enterokinase treated sample (~21.6 kDa) is shown on gel.
Structural analysis of MAGE-A4 using circular dichroism (CD) spectra
To get further insights into the stability, dimerization and structure of MAGE-A4, I purified various forms of MAGE-A4 protein and analysed them using Circular Dichroism.
I prepared four different samples. Monomer with full length tag 48aa - (sample M); dimer with full length tags (Sample D); monomer without tag (Sample MA) and dimer without tag (Sample MB). The majority of the protein contained α-helices and β-sheets while only aminor fraction was comprised of turns (Table 1). However, the secondary structures of different MAGE-A4 forms differed (Figure 9).
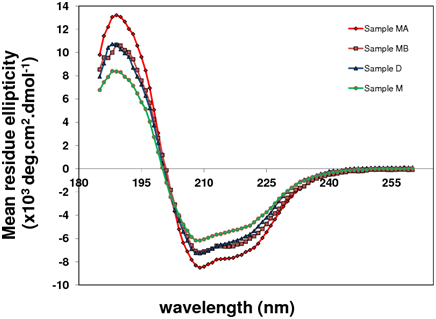
Figure 9 MAGE-A4 variants studied at 25 °C; (Sample M) monomer with full length tag 48 aa, (Sample D) dimer with full length tags, (Sample MA) monomer without tag and (Sample MB) dimer without tag.
Protein Sample |
Helix (%) |
Sheet (%) |
Turn (%) |
Others (%) |
Sample M |
18.2 |
29.2 |
9.4 |
37.7 |
Sample D |
30.2 |
29.3 |
13.6 |
32.1 |
Sample MA |
25 |
27.7 |
12.8 |
34.7 |
Sample MB |
25.9 |
26 |
10.6 |
33.8 |
Table 1 Clinical and biochemical variables of individuals with overweight-obesity
SD: Standard Deviation; BMI: Body Mass Index; WC: Waist Circumference; AC: Abdominal Circumference; HC: Hip Circumference; RER: Respiratory Exchange Ratio; HR: Hear Rate.
From the secondary structure analysis of various MAGE-A4 forms we see a clear difference. In contrast to the respective dimers there is a big difference between monomer with and without tag. Due to stability and solubility difficulties we were unable to obtain sufficient amount of samples, except in the case of the monomer with tag. Further analysis of structural studies is required to reveal the role of the tag.
The CD spectra reveal not only the effect of the tag on the structure of the MAGE domain but also the intrinsically disordered nature of the protein. MAGE-A4 could be an intrinsically disordered protein like other cancer/testis antigens, characterized by a lack of stable tertiary structure. In the case of dimers the transition from disorder to order could happen following favourable interactions.33
We also measured melting temperatures (Tm) for monomer and dimer forms after cleavage of tags. Tm is around 45 and 41°C, respectively. Melting temperature of 41°C was also experimentally determined for MAGE-A4 with full length tag (Figure 10). For such proteins with low Tm, it is hard to do NMR measurements or to perform purification at room temperature. So I purified and stored the proteins always at temperatures around 4°C. CD spectra can be used for MAGE domain secondary and tertiary structure identification.

Figure 10 Melting temperatures (Tm) of MAGE-A4 (Sample M) measured using CD. Unfolding of MAGE-A4 monomer with long tag between 37-45 °C was observed.
MAGE domain study using NMR
The MAGE-A4 stability test forNMR: To improve the stability of the MAGE-A4 protein we employed stability test using various buffers in 96-well format (Appendix 2.7.3). Various conditions were examined by changing pH, salts, stabilising chemicals and amino acids in various buffer solutions.
MES 4% jeffamine (pH 6)>>MES 2% jeffamine+50mM CsCl+50mM glutamic acid (pH 6)>>Tris 4% jeffamine+50mM CsCl (pH 7.5).
The above conditions are more optimal for MAGE-A4 protein. Organic buffers are good but are expensive and more difficult to use for NMR. All PBS buffer samples show moderate or low stability at pH 7.34. However, PBS buffer is biologically relevant and useful for NMR, so we chose 1X PBS for NMR studies.34
To increase the stability of monomeric units, low concentration of sample, storage at low temperatures from 4 to 10°C, decreasing ionic strength by low salt in 1X PBS, and decreasing hydrophobic interactions by adding 5-30% glycerol to 1X PBS, help all increase protein stability.
MAGE-A3 was well expressed, but it was unstable (makes aggregates in a short time). MAGE C2 is stable but its expression was low. MAGE-A4 shows moderate stability and good expression. So we selected A4 and C2 for further studies.
Structural Analysis using NMR Method: Ammonium chloride labelled with 15N isotope is relatively inexpensive, and the 1H-15N HSQC is a sensitive experiment whereby a spectrum can be acquired in a relatively short time. 1H-15N HSQC is, therefore, often used to screen candidates for their suitability for structure determination by NMR, as well as optimization of sample conditions.
In general, each residue would produce an observable peak in the spectra, with the exception of proline which lacks an amide proton. In a typical HSQC spectrum, the NH2 peaks from the side chain of asparagine and glutamine appear as doublets in the top right corner. The side chain amide peaks from tryptophan are usually shifted downfield and located near the bottom of the left corner. The backbone amide peaks of glycine normally appear near the top of the spectrum. In order to check whether MAGE-A4 is well suited for NMR Spectroscopy, the 1H-15N HSQC spectrum was acquired using a 15N-labeled protein. In this spectrum, the peaks are well-dispersed meaning that the MAGE-A4 protein is folded, and most of the individual peaks can be distinguished. The number of peaks was close to the expected number of peaks based on the amino acid sequence (Figure 11). The tag part of the recombinant protein is often unfolded and flexible, being thus expected to be invisible in the HSQC spectrum.
The assignment of the spectrum is essential for a meaningful interpretation of more advanced NMR experiments such as structure determination and relaxation analysis. The quality of the obtained spectrum shows that NMR study of MAGE-A4 is feasible. 1H-15N HSQC NMR spectra of MAGE-A3 and MAGE-C2 proteins looked similar to MAGE-A4.
Limitations for MAGE Domain Structural Study Using NMR: An NMR protein sample should be stable for 2weeks at 30°C. MAGE-A4 protein is stable at lower temperatures for a longer time (up to 2 months at 4°C). However, our tests suggested relatively low melting temperature and low stability (Figures 10 & 12). It is difficult to analyse the structure of such sensitive proteins as it is hard to keep protein stable at higher temperatures. In addition, MAGE domain may assemble into dimers and aggregates which can also complicate the spectral assignment due to peak shift.

Figure 12 Temperature stability test (1D spectra of MAGE-A4). Temperature stability test was done using NMR spectrophotometer. When we incubated the sample at various temperatures we observed protein unfolding and signal loss due to sample degradation and precipitation. Similar fractions were analyzed on a 15% SDS-PAGE gel (top right panel). Green arrow points to fresh sample stored at 4 °C, red arrow points to sample at 30 °C for an hour, black arrow points to sample at 20 °C for 3 weeks, degraded protein.
NMR titration experiment: MAGE-C2 Vs EID2: The 1H-15N HSQC experiment is also useful for mapping the binding interface in protein-protein interactions. I planned to study the interaction between human orthologs of NSE3 and NSE4 (Appendix 2.7.4). So I tested interactions between MAGE-C2 and EID2 peptide (NSE3 and NSE4 orthologs, respectively). MAGE-C2 was purified following the same methods as MAGE-A4. Moderate changes in the chemical shifts of some peaks were observed, and these peaks are likely to lie on the binding surface where the binding perturbed their chemical shifts (Figure 13). However, the EID2 (QRNPHRVDLDILTFTIALTASE) peptide precipitated rapidly during titration. This may explain the modest chemical shift changes observed. Interactions between MAGE-C2 and EID2 were then analysed and proved using yeast two hybrid tests and ELISA pepscan.35
MAGE domain structure and its interaction with EID proteins was studied. Our primary goal was to solve MAGE domain structure and understand its interactions with the EID protein. Structural modelling together with experimental data showed how the core helical region of the NSE4/EID domain binds into the conserved pocket characteristic of the MAGE protein family. The conservation and binding of the interacting surfaces suggests tight co-evolution of both Nse4/EID and Nse3/MAGE protein families.
I have developed purification protocols for the MAGE-A3, MAGE-A4 and MAGE-C2 proteins. We have tried to solve the MAGE domain structure of MAGE-A4 using NMR method. Due to the precipitation and instability of our proteins we were not able to assign the structure. We tried to explore MAGE-C2-EID2 interaction with the expectation that the binding partner will stabilize the MAGE protein. However, the EID2 peptide used in our approach precipitated as well and did not provide detailed information about the MAGE-C2-EID2 interaction.
None.
The author declares no conflict of interest.

©2016 Bathula. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.