MOJ
eISSN: 2374-6920


Research Article Volume 3 Issue 2
1Department of Pathology, Sylhet Agricultural University, Bangladesh
2College of Veterinary Medicine and Bio-Safety Research Institute, Chonbuk National University, South Korea
Correspondence: Md. Masudur Rahman, Department of Pathology, Faculty of Veterinary and Animal Science, Sylhet Agricultural University, Sylhet 3100, Bangladesh, Tel +880821761002(201), Fax +880821761980
Received: December 10, 2015 | Published: February 5, 2016
Citation: Rahman MM, Badruzzaman ATM. Cloning and expression of mature chicken interleukin-18 using live attenuated Salmonella enterica serovar typhimurium. MOJ Proteomics Bioinform. 2016;3(2):28-32. DOI: 10.15406/mojpb.2016.03.00079
Cytokines may represent natural and environmental friendly alternatives to existing conventional disease control strategies. However, the practical use of chicken cytokines in controlling avian diseases is limited due to the lack of cost effective production and delivery systems to enable mass administration. In an effort to provide valuable insight into the use of attenuated Salmonella enterica serovar Typhimurium as a delivery vector for chicken cytokines we amplified and cloned chicken interleukin-18 (chIL-18) genes and constructed pYA3560 plasmid vector encoding chIL-18, which is specially designed for Salmonella enterica serovar Typhimurium bacteria. Subsequently, chIL-18 encoding pYA3560 plasmid was electroporated into the Salmonella enterica serovar Typhimurium and expression of chIL-18 protein and it’s bioactivity were checked by immunoblot analysis and Griess assay respectively. Identity of chIL-18 nucleotide and amino acid sequences to the sequences of other species was also evaluated. According to our results, attenuated Salmonella enterica serovar Typhimurium might be an excellent delivery vector for chicken cytokines. To use Salmonella enterica serovar Typhimurium as delivery vector of chicken cytokines, their ability to synthesize active cytokine in chicken host system needs to be well addressed.
Keywords: salmonella, delivery vector, chicken interleukin-18, cloning and expression
IL-18, interleukin-18; Asd, aspartate β-semialdehyde dehydrogenase; DAP, diaminopimelic acid; TGEV, transmissible gastroenteritis virus; PrV, pseudorabies virus; RT-PCR, reverse transcription-polymerase chain reaction; TCA, trichloroacetic acid; IgG, immunoglobulin G
Cytokines play pivotal roles as natural mediators and regulators of the immune response1 and therefore may offer exciting new alternatives over existing conventional control measures which include combined use of vaccines, antibiotics and chemicals. The utilization of chicken cytokines in poultry disease prevention is becoming more promising with the growing list of new cytokines.2 Interleukin-18 (IL-18) provides an important link between innate and adaptive immunity through the induction of IFN-γ secretion.3 Chicken interleukin-18 (chIL-18) has been shown to play a significant role in inducing antiviral immune responses against several viral infections including H5-H7 AIV.4 However, there have several critical limitations in the use of chicken cytokines in disease prevention which include effective delivery system, cost effective commercial production, protein stability, bioactivity in vivo etc. Recent developments in recombinant DNA technologies and gene delivery vectors provide realistic approach in the use of recombinant chicken cytokines in disease prevention.
Live attenuated Salmonella vaccine strains have been used as carriers of heterologous antigen(s) from bacteria, viruses and parasites.5 Following oral administration, Salmonella has been shown to be capable of stimulating systemic antibody and cell-mediated immunity.6,7 Conventionally, a Salmonella vaccine strain contains a plasmid-based expression vector, which encodes the heterologous antigen(s) of interest, and an antibiotic-resistance selection marker that is used, after addition of the corresponding antibiotic, for plasmid maintenance. The use of such Salmonella strains has been discouraged because of concerns over safety regarding use in humans, and because of concerns regarding cost-effectiveness.
The attenuated S. enterica serovar Typhimurium strain x8501 harbours deletion mutations in cya and crp, defective in the synthesis of the adenylate cyclase and cyclic AMP receptor, and asd, which encodes the aspartate β-semialdehyde dehydrogenase (Asd), an essential enzyme for cell-wall biosynthesis.8 This Asd auxotrophic mutant was unable to grow in complex medium without supplementation with diaminopimelic acid (DAP), a bacterial amino acid not found in eukaryotes, but, after trans-complementation with an Asd+ plasmid, the mutant’s growth was restored.9 Hence, only Asd+ plasmid-carrying cells can grow in DAP-free medium, making the Asd- Salmonella strain dependent on the plasmid maintenance, owing to the balanced lethal relationship between vector and host systems.10 Recently, several multicopy, stable Asd+ antigen-expressing vectors (e.g. pAY3493, pAY3560) has been specially designed to express recombinant protein antigens by means of the fusion of the β-lactamase signal sequence in an Asd- Salmonella vaccine strain11 that makes it an unique gene delivery vector. Recently, we showed that oral administration of attenuated S. enterica serovar Typhimuirum harboring swine cytokine genes (swIFN-α, swIL-18 genes) could effectively express biologically active cytokines (swIFN-α, swIL-18) in piglets that could alleviate the clinical severity induced by the transmissible gastroenteritis virus (TGEV) and pseudorabies virus (PrV).12,13 Here, efficiency of attenuated S. enterica serovar Typhimuirum in vitro expressing bioactive non-mammalian cytokine, chIL-18, has been studied in order to circumvent critical barrier in using chicken cytokine in disease prevention.
Bacterial strains, plasmids, media, and growth conditions
Escherichia coli χ6212 (F-λ-ϕ80Δ (lacZYA-argF) endA1 recA1 hsdR17 deoR thi-1 glnV44 gyrA96 relA1 ΔasdA4)10 was used as the host strain for construction of the Asd+ plasmid vectors encoding chIL-18. Attenuated S. enterica serovar Typhimurium χ8501 (hisG Δcrp-28 ΔasdA16), provided by Dr H.Y. Kang (Pusan National University, Korea)11 was used as the host bacteria to express chIL-18 protein. The pYA3560 Asd+plasmid was derived from pYA3493 Asd+ plasmid by replacing the pBR ori gene (origin of replication of pBR322 plasmid) with the p15A ori gene (origin of replication of p15A plasmid) to maintain stability in bacteria.11 E. coli and S. enterica serovar Typhimurium cultures were grown at 37°C in Lennox broth, Luria-Bertani (LB) broth, or on LB agar. Diaminopimelic acid (DAP; Sigma-Aldrich, St. Louis, MO, USA) was added (50μg/ml) to induce the growth of Asd-negative bacteria.10 Phosphate-buffered saline (PBS, pH 7.4) containing 0.01% gelatin (BSG) was used for the resuspension of Salmonella bacteria that were concentrated by centrifugation at 7000 × g at 4°C for 5min.
Cloning of chIL-18 gene in pYA3560 plasmid vector
Chicken splenocytes (107cells/ml) were prepared in complete RPMI medium and stimulated with lipopolysaccharide (LPS, 20μg/ml) for 48h. Following stimulation the cells were harvested and total RNA was extracted from the harvested cells which were used to amplify the chIL-18 gene by reverse transcription-polymerase chain reaction (RT-PCR) using specific primer pairs (Table 1). The PCR products were then inserted into pGEM-T vectors (Promega, Madison, WI, USA) and the chIL-18 gene was sequenced to confirm the authenticity of the inserted sequences. Subsequently, chIL-18 gene was subcloned into pYA3560 plasmid vector. The pGEM-T vector encoded with chIL-18 gene was digested with EcoRI and HindIII after which the released fragment containing the chIL-18 gene was inserted into the same restriction site of pYA3560 plasmid vector using E. coli χ6212 host grown in the presence of DAP. The positive colonies of E. coli χ6212 harboring chIL-18-encoding pYA3560 vector were selected in the absence of DAP.
Target Gene |
|
Primer Sequence (5’-3’) |
Accession No. |
chIL-18a |
Fb |
GAATTCGCC TTTTGTAAGGATAAAACT |
FJ882408.1 |
Rb |
AAGCTTTCATAGGTTGTGCCTTTC |
Table 1 Primers for reverse transcription and PCR amplification of mature chIL-18 gene
aThe primer pair specific for chIL-18 gene was designed using mature chIL-18 nucleotide sequences (Genebank accession number FJ882408.1), and the sequences of two primers were checked using the NCBI Blast Software.
bThe forward and reverse primers specific for chIL-18 gene contain EcoRI and Hind III restriction sites as indicated by the underline.
Construction of attenuated enterica serovar Typhimurium expressing chIL-18
To construct attenuated S. enterica serovar Typhimurium expressing chIL-18, S. enterica serovar Typhimurium χ8501 (1×108 colony-forming unit (cfu)) washed extensively with sterilized ice-cold WB (10% ultra pure glycerol and 90% distilled water, v/v) were mixed with 10 pg to 0.1μg of chIL-18-encoding pYA3560 plasmid DNA on ice in a 0.2-cm cuvette and electroporated using a Bio-Rad Gene Pulser at 12.5 kV/cm (2.5 kV, 25μF and 200Ω; Bio-Rad, Hercules, CA, USA). The bacteria were then removed from the cuvette into sterile culture tubes containing 1ml of LB broth medium and incubated with moderate shaking for 60min at 37°C. The transformed cultures (each 100μl) were then plated onto LB agar plates in the absence of DAP. Finally, colonies of attenuated S. enterica serovar Typhimurium harboring chIL-18-encoding pYA3560 (χ8501/chIL-18) vector were cultured and stored after confirmation of the coding sequences.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analyses
The expression of chIL-18 protein by S. enterica serovar Typhimurium harboring chIL-18-encoding pYA3560 (χ8501/chIL-18) plasmid was identified by immunoblotting following gel separation of prepared proteins by SDS-PAGE. For the preparation of protein samples, Salmonella bacteria cultured for 12, 18, and 24h were resuspended in 4ml of 20mM Tris-HCl (pH 8.6) and then disrupted by two passages through a French pressure cell (American Instrument, Silver Spring, MD, USA). Cell lysates were centrifuged at 7000 ×g at 4°C for 6min to remove unbroken cells. The supernatant fraction was used for protein samples of cell lysates. The original culture supernatants were passed through a 0.22-μm filter and protein that had been secreted into the supernatant was precipitated with 10% trichloroacetic acid (TCA) at 4°C for 1h. Prepared protein samples were boiled for 5min and then separated by SDS-PAGE. The protein bands were visualized by staining with 0.1% Coomassie brilliant blue R-250 solution (ELPIS-Biotech Inc., Daejeon, Korea). For immunoblotting, the resolved protein was transferred electrophoretically to nitrocellulose membrane. The membrane was then blocked with a blocking buffer consisting of PBS, 3% skim milk, and 0.5% Tween 20, and incubated with 6xHis-Tag antibody (Novagen, Madison, WI, USA) to detect 6×histidine tagged-chIL-18. Following 1.5h incubation, peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) (Southern Biotech, Birmingham, AL, USA) was added. Immunoreactive bands were detected by the addition of chemiluminescence dye using a WEST-one™ Western Blot Detection System (iNtRON, Seongnam-Si, Korea) in the presence of hydrogen peroxide.
Bioassay of recombinant chicken IL-18
In order to analyze the biological activity of recombinant chicken IL-18 on the induction of IFN-γ, normal spleen lymphocytes (SPL) (5×106/ml) isolated from 3-week-old SPF chickens were incubated in Iscove’s Modified Dulbecco’s Media (IMDM, Sigma), supplemented with 10% FBS, 100U/ml of penicillin, and 100mg/ml of streptomycin with chIFN-γ (control), or S. enterica serovar Typhimurium-expressed chIL18 (60, 120, 250 and 500ng/ml) for 72h in 96-well plates at 41°C in a 5% CO2 incubator. Supernatants from these cultures were added to HD11 culture and NO production was measured after 48h of incubation by Griess assay as described previously.13 The control media (plain IMDM) served as a negative control and COS7 cell-expressed IFN-γ as a positive control. IL-18 alone (1000ng/ml) was also used as a negative control.
Determining the identity of chicken IL-18 sequence to the sequences of other species
In order to determine the identity of chicken IL-18, nucleotide and amino acid sequences of mature chicken IL-18(FJ882408.1), swine IL-18(NM_213997.1), bovine IL-18(NM_174091.2), canine IL-18(NM_001003169.1), mouse IL-18(AY362457.1) and human IL-18(AF077611.1) were collected from NCBI GenBank repository and subjected to analyze using EMBOSS Needle (EBI) software.
In order to construct pYA3560 plasmid, chIL-18 gene (510bp) was amplified by RT-PCR and amplified PCR products were gel electrophoresed (Figure 1). The PCR products were then inserted into pGEM-T vector and the chIL-18 gene was sequenced to confirm the authenticity of the inserted sequences (data not shown). Subsequently, chIL-18 gene was subcloned into the Eco RI and HindIII sites of pYA3560 plasmid that was used for the expression of chIL-18 in S. enterica serovar Typhimurium (Figure 2). In order to construct attenuated S. enterica serovar Typhimurium chIL-18-encoding pYA3560 plasmid vector was subsequently transformed into attenuated S. enterica serovar Typhimurium χ8501 hosts by electroporation and positive colonies of S. enterica serovar Typhimurium χ8501 harboring chIL-18-encoding pYA3560 (χ8501/chIL-18) was selected in the absence of DAP. The in-frame fusion of chIL-18 with the β-lactamase signal sequence was confirmed by nucleotide sequencing (data not shown).
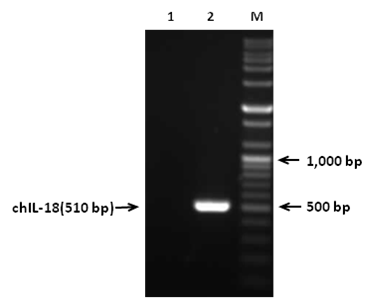
Figure 1 RT-PCR amplification of mature chIL-18 gene. Total RNAs were extracted from LPS-stimulated splenocytes and used to amplify the chIL-18 gene by RT-PCR using specific primer pairs. Lane M, size marker;
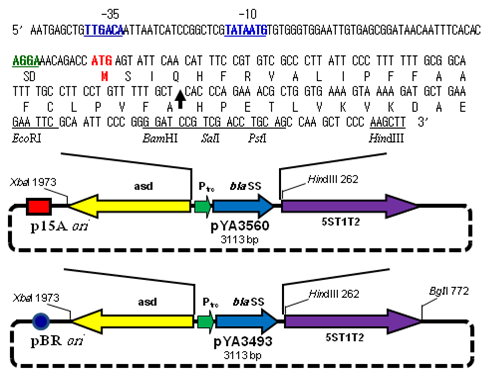
Figure 2 Diagram of periplasmic secretion Asd+ vector pYA3560 and pYA3493.
The pYA3560 Asd+plasmid was derived from pYA3493 Asd+ plasmid by substituting the pBR ori gene with the p15A ori gene. A DNA fragment encoding the β-lactamase signal sequence and 12 amino acid residues of the N terminus of mature β-lactamase of plasmid pBR322 was positioned under the control of the Ptrc promoter. A map of the pYA series vectors (pYA3560 and pYA3493) and the nucleotide sequences of the Ptrc promoter region, β-lactamase signal sequence (bla SS) and multicloning sites are shown. The Ptrc sequences for -35, -10 (RNA polymerase-binding site), and Shine-Dalgarno box (SD, ribosomal binding site) are indicated by blue and green boldface, and the translocation start codon (ATG) is in red boldface. An arrow within the sequence indicates the signal peptidase cleavage site. Unique restriction enzyme sites in the multicloning site are indicated, and 5ST1T2 is a transcriptional terminator.
To identify the expression of chIL-18 protein by transformed S. enterica serovar Typhimurium, TCA-precipitated culture supernatants and bacterial cell lysates prepared at different incubation time points (12, 18, and 24h) were subjected to SDS-PAGE and immunoblot analysis. Attenuated S. enterica serovar Typhimurium harboring the empty vector pYA3560 (χ8501/ pYA3560) cultured for 18 h was used as a negative control. The expression of chIL-18 protein from S. enterica serovar Typhimurium harboring chIL-18-encoding pYA3560 (χ8501/chIL-18) was detectable as early as 12h post-incubation, and gradually increased, saturating the culture supernatants and cell lysates within 24h of incubation (Figure 3). Furthermore, the biological activity of secreted chIL-18 protein in culture supernatant was evaluated in terms of the production of NO by HD11 cells stimulated with culture supernatant containing recombinant chIL-18 measured by Griess assay. When the supernatants of spleen cells, which were stimulated with recombinant IL-18 for 72h were added to HD11 cells and incubated for 48 h, the production of NO was significantly raised at doses between 60 and 120ng/ml of recombinant S. enterica serovar Typhimurium-expressed IL-18 protein (Figure 4). IL-18 alone, which was used as the negative control, did not induce NO. Taken together, these results indicate that the attenuated S. enterica serovar Typhimurium harboring chIL-18-encoding pYA3560 (χ8501/chIL-18) successfully expressed bioactive chIL-18 protein, which was actively secreted into the culture media rather than as the result of nonspecific membrane leakage or cell death by lysis. The identity of chicken mature IL-18 nucleotide (nt) and amino acid (aa) sequences to the counter sequences of other species were evaluated (Table 2). Analysis of nt and aa sequences of cloned mature chIL18 showed 100% identity with the GenBank sequence of chIL-18 both at nt and aa level. Cloned mature chIL-18 showed 52%, 53%, 50%, 51% and 51% identity with swIL-18, boIL-18, caIL-18, moIL-18 and huIL-18 at nucleotide level and 31%, 32%, 30%, 30% and 34% identity at amino acid level respectively.
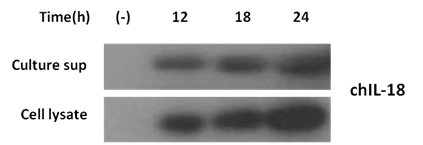
Figure 3 Identification of chIL-18 expression from constructed S. entericaserovar Typhimurium by immunoblot analysis. The chIL-18 protein expressed by χ8501/chIL-18 were detected from both TCA-precipitated culture supernatants (sup) and cell lysates by immunoblotting with 6´His-Tag antibody after 12, 18, and 24h incubations. Attenuated S. entericaserovar Typhimurium carrying empty vector pYA3560 (χ8501/ pYA3560) or pYA3493 (χ8501/ pYA3493) cultured for 18 h was used as a negative control.

Figure 4 Induction of nitric oxide (NO) production by rChIL-18 induced IFN-γ. The biological activity of recombinant chIL-18 was assayed by stimulating primary splenocytes with chIFN-γ (control), or S.enterica serovar Typhimurium -expressed chIL18 for 72h. Supernatants from these cultures were added to HD11 culture and NO production was measured after 48h of incubation. The control media (plain IMDM) served as a negative control and COS7 cell-expressed IFN-γ as a positive control. IL-18 alone (1000 ng/ml) was also used as a negative control. Doses of recombinant chIL18 tested were 60, 120, 250 and 500 ng/ml. Values with different superscripts denote significant difference at p< 0.05.
|
chIL-18a |
swIL-18 |
boIL-18 |
caIL-18 |
moIL-18 |
huIL-18 |
chIL-18 |
100(100)b |
52(31) |
53(32) |
50(30) |
51(30) |
51(34) |
swIL-18 |
- |
100(100) |
92(89) |
92(88) |
72(62) |
84(75) |
boIL-18 |
- |
- |
100(100) |
90(83) |
73(64) |
85(77) |
caIL-18 |
- |
- |
- |
100(100) |
72(63) |
84(73) |
moIL-18 |
- |
- |
- |
- |
100(100) |
72(61) |
huIL-18 |
- |
|
- |
- |
- |
100(100) |
Table 2 Identity of the chicken mature IL-18 sequence to the sequences of other species at the nucleotide (nt) and amino acid (aa) levels
achIL-18, mature chicken IL-18 (FJ882408.1); swIL-18, mature swine IL-18 (NM_213997.1); boIL-18, mature bovine IL-18 (NM_174091.2); caIL-18, mature canine IL-18 (NM_001003169.1); moIL-18, mature mouse IL-18 (AY362457.1); huIL-18, mature human IL-18 (AF077611.1).
bThe numbers outside the brackets indicate the % similarities at the nt levels; the numbers inside the brackets indicate the % identities at the aa levels.
Attenuated S. enterica serovar Typhimuirum is a well-characterized vaccine strain available to livestock industry for the prevention of Salmonellosis. This registered attenuated Salmonella strain has the potential for heterologous protein delivery in livestock vaccination.14 In our study, we used Asd+ plasmid DNA (pYA3560) that are retained in vivo in Salmonella vaccine strains devoid of the asd gene, as an essential factor for a balanced-lethal host-vector system.10,11 A signal sequence plus an additional 12 amino acids of mature β-lactamase are required to translocate β-lactamase through the cytoplasmic membrane of gram-negative bacteria.15,16 Thus, fusion of a protein to the β-lactamase signal peptide is expected to promote the secretion of the fusion protein into the bacterial periplasm.16,17 For the latter issue, the presently-constructed pYA3560 plasmid DNA was designed to use for the periplasmic secretion of chIL-18 by the Salmonella vaccine. We reasoned that chIL-18 attached to the β-lactamase signal peptide should be secreted into the culture of attenuated Salmonella bacteria. Indeed, a significant amount of chIL-18 protein was secreted into the culture supernatant, as detected by Western blot (Figure 3). As chIL-18 secreted from χ8501/chIL-18 was shown to induce nitric oxide (NO) production by HD-11 cells measured by Griess assay indicating IFN-γ release, the secreted chIL-18 was biologically active. Additionally, in vivo biological activity of chIL-18 secreted by χ8501/chIL-18 in chicken host system has been studied at our lab.18–21
Cytokines, as natural mediators of the innate and adaptive immune responses, may be an excellent alternative to conventional therapeutics such as treatment with antibiotics. Indeed, cytokine therapy has been shown to be effective in livestock and poultry. The use of chicken cytokines is becoming more feasible with the recent cloning of a number of cytokine genes, since the chicken’s immune system is similar to that of mammals. However, the main obstacles in the practical use of chicken cytokines for prevention and/or therapeutic of avian diseases are the lack of suitably cost-effective production and delivery systems for mass administration. Here, we have provided valuable insight into the use of attenuated Salmonella bacteria as delivery vector of chIL-18 to ensure mass administration, thereby overcoming the cost and production issues relating to the use of chicken cytokines. Based on our findings, we recommend that attenuated Salmonella enterica serovar Typhimurium might be an excellent candidate of delivery vectors for chicken cytokines.
This study was supported by the Mid-career Research Program (2010-0000134, 2010-0029108) through the National Research Foundation of Korea (NRF) funded by the ministry of Education, Science, and Technology. This study was supported in part by grant No. RTI05-03-02 from the Regional Technology Innovation Program of the MOCIE. The authors thank Dr H.Y. Kang (Pusan National University, South Korea) for supplying the pYA3493 and pYA3560 vectors and Dr. Seong Kug Eo (Chonbuk National University, South Korea) for critically reviewing the draft manuscript.
The author declares no conflict of interest.

©2016 Rahman, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.