MOJ
eISSN: 2374-6920


Mini Review Volume 1 Issue 5
Pillar of Engineering Product Development, Singapore University of Technology & Design (SUTD), Singapore
Correspondence: Rajesh Chandramohanadas, Asst. Professor, Pillar of Engineering Product Development, Singapore University of Technology & Design, 20 Dover Drive, Singapore, Tel +65 64994632, Fax +65 6779 5161
Received: November 11, 2014 | Published: December 6, 2014
Citation: Subramanian G, Sinha A, Thu Trang CT, et al. Chemical biology and proteomics for hunting drug targets for human malaria: an update. MOJ Proteomics Bioinform. 2014;1(5):134-137. DOI: 10.15406/mojpb.2014.01.00031
Chemical biology approaches combined with proteomics provide a powerful tool to dissect biological mechanisms with high precision, accuracy and temporal resolution. In the recent years, infectious disease biology, in particular, translational research to discover new drug targets for human diseases has benefited immensely by leveraging this approach. Identification of novel drug targets is critical to combat infections, such as human malaria. Malaria parasites tend to acquire drug resistance very rapidly warranting the design of new lines of chemotherapy. In this article, we briefly review recent discoveries in the field of malaria research that utilizes chemical biology and proteomics targeted at identifying viable drug scenarios and potential drug targets.
Keywords: malaria, chemical biology, activity-based probes, proteomics, target identification, drug discovery, plasmodium falciparum
Malaria is among the deadliest and wide-spread infectious diseases. Although, there has been significant progress in malaria prevention and control through chemotherapeutic approaches and vector control initiatives, half of the world’s population still inhabits in malaria endemic regions.1 The situation is even more alarming due to the emergence of rapid parasitic resistance against commonly used drugs.2–5 Hence it has become all the more important to design new ways of tackling this deadly infection. Among several variants of plasmodial parasites infecting humans, the most serious pathological condition is caused by Plasmodium falciparum infections. Asexual intra-erythrocytic developmental processes, primarily steps associated with hemoglobin degradation, parasite invasion or parasite release are known to be viable checkpoints for potential chemotherapy development. Identification and detailed exploration of such scenarios requires highly robust and easily adaptable methodologies. Functional proteomics approaches using small molecule inhibitors and Activity-Based Probes (ABPs), present an ideal platform to temporally dissect essential parasitic survival mechanisms against which chemotherapy may be developed.
Among the many antimalarial drugs currently used, quinolones and artesunate constitute the most widely used and effective.6,7 However, due to the emergence of resistant forms of parasites against these commonly used drugs, identification of novel drug targets is urgently warranted.8,9 During the intra-erythrocytic developmental cycle, plasmodial parasites engage in highly regulated interactions with its host cells to facilitate entry, survival, replication and eventual release. Detailed understanding of these life-stage events using genetic, biochemical or chemical tools will provide new avenues for potential chemotherapy development against malaria. Such processes during the developmental cycle that lasts about 48 hours are unique and are mediated by specific sets of effector proteins that are either expressed or activated only at the required life stages. Most of these proteins are essential for parasitic survival and cannot be deleted genetically limiting the application of genetic manipulation techniques to study them in greater detail.
In contrast, small molecule inhibitors capable of altering a biological pathway without interfering with transcriptional or translational machineries provide an efficient tool to (a) selectively manipulate the biological event, (b) generate chemical phenotypes that can be studied using alternate methods and (c) directly identify/validate the target molecules that orchestrate these processes. To do this, small molecule libraries that are designed to influence the desired life-stage are tested on cultured parasites to evaluate their efficacy and to identify possible phenotypes. Once phenotypic characterization is completed, chemically modified forms of the potent scaffolds are developed as activity-based probes (ABPs). These probes maintain the active functional groups required for its inhibitory potential, however, also carry additional reporter moieties to enable functional read outs of inhibition, linked through appropriate chemical linkers (Figure 1). These reporter moieties aid monitoring of the sub-cellular targets in real time. Although, fluorophores such as BODIPY or TAMRA10–12 are used widely as reporters, biotinylated molecules13 are the most preferred as they allow the affinity purification/identification of the targets using streptavidin-based affinity purification methods. Such approaches have widely been used in the study of malaria biology, in relevance to host invasion, parasitic progression/replication as well as merozoite egress as represented in Figure 1. This review describes the novel drug targets unraveled through such approaches.
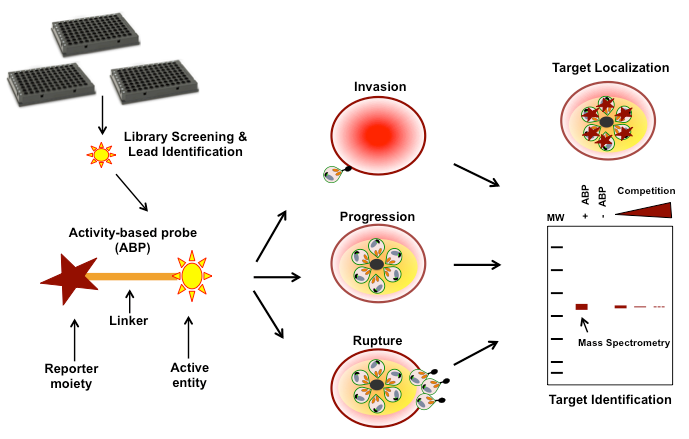
Drug targets relevant to invasion
Red blood cell invasion by plasmodial parasites is rapid and often completed in less than a minute. The process consists of distinct interaction steps between invading merozoites and their host cell surface in a concerted effort of sugar-binding proteins, adhesins and proteases. Among the several plasmodial surface proteins, MSP-1 is the most abundant protein on the merozoite surface14–16 and is known to be one of the primary mediators of merozoite invasion. It is synthesized as precursor protein, which undergoes proteolytic processing during merozoite egress. During invasion, this complex is further fragmented and only its C-terminal portion remain attached to the parasite surface whereas the remaining portions are shed off. Antibodies targeting various domains of MSP-1 have been shown to effectively block invasion,17 although they lack therapeutic potential due to poor permeability. Hence, inhibitors interfering with MSP-1 processing represent highly promising drug candidates.18,19 A number of sugar derivatives have been shown to bind to MSP-1 and block invasion, highlighting an opportunity to target this protein for antimalarial development. Recently, heparin was shown to inhibit MSP-1 processing by binding to one of its subunit called MSP-133, thereby inhibiting invasion. Using affinity purification approaches, Boyle et al.,20 were able to demonstrate specific binding to heparin of MSP-133, and not MSP-119 to heparin. More recently, combining chemical biology, computational biology and biochemistry, our group described the identification of a small molecule, NIC that was able to bind to the shorter C-terminal fragment of MSP-1 (MSP-119). Small molecules evaluated in this study were initially selected through virtual screening of compounds with potential binding affinity to MSP-119. Subsequently, detailed chemical biology experiments in combination with biochemical as well as proteomics-based validation confirmed specific and direct binding of NIC to plasmodia MSP-119. Our results revealed that binding of NIC to MSP-119 resulted in reduced invasiveness of merozoites derived from P. falciparum and P. vivax.21 These results represent a major advancement in the field of antimalarial development targeted against invasion proteins, since the residues within MSP-119 that binds to NIC are significantly conserved across multiple plasmodia, highlighting the possibility to explore such scaffolds for pan-antimalarial development.
Parasite invasion also involves shedding and presentation of surface proteins such as MSP-1 and AMA-1,22,23 which appears to be driven by serine proteases of the subtilisin family. Among such enzymes, Subtilisin-1 like protease (Sub-1) was shown to be proteolytically active during both invasion and egress. Sub-1 is capable of processing a variety of parasite surface proteins such as MSP1/6/7 and members of a family of papain-like putative proteases called SERA,24 thereby aiding invasion and egress.25,26 Recent research demonstrates the targeted development of novel α-ketoamide based molecules as well as quinolylhydrazones27 as powerful inhibitors of this enzyme. Another much studied sheddase; subtilisin-2 like protease of the malaria parasite appears to process invasion proteins such as AMA-1.28 The important cysteine proteases such as falcipain-2 and falcipain-3 are validated as potential targets upon which innovative classes of new drugs are being designed.29–31
Rupture mediators as drug targets
Application of chemical biology and proteomics has played an important role to discover various parasitic and host proteases involved in host cell rupture thereby releasing plasmodial merozoites. Blocking the activation of such proteases irreversibly locks mature merozoites within the host cells preventing a further cycle of infection. Serine Repeat Antigens (SERAs) play a critical role in egress pathway32 and this in turn is facilitated by upstream active proteases such as PfSUB133 and DPAP3. Inhibitors of DPAP3 and PfSUB1 blocked the processing of SERAs 5 & 6 thereby blocking egress,34 showcasing the role of these proteases in triggering host cell collapse and thus, their potential as drug targets. In addition, studies using inhibitors of papain family cysteine proteases and corresponding ABPs have demonstrated the importance of host calpain activation during latter stages of parasite development35 in Plasmodium. Calpain activation resulted in irreversible cytoskeletal damage in the erythrocyte and thereby aided parasite release.36 Furthermore, this study provided direct evidences depicting both Plasmodium and Toxoplasma confiscates calpain proteases to facilitate parasite egress and that silencing host calpain-1 activity, either biochemically or genetically, resulted in impaired parasite exit. Development of host calpain specific inhibitors will be a new avenue for antimalarial development leveraging on host cell pathways required for parasitic replication.35,37,38
Other emerging targets
Digestive vacuole enzymes have been extensively explored as candidate drug targets against malaria.39 Recent investigations also highlight the possibility to approach a number of other proteins critical for parasite survival and replication as viable targets for novel chemotherapy development. For instance, spiroindolones that targets a P-type cation-transporter ATPase 4 (PfATP4) was shown to be effective against P. falciparum and P. vivax malaria40,41 and compounds of that lineage are currently undergoing clinical validation. N-myristoyltransferase, an enzyme required for the myristoylation of several plasmodial proteins appears to be a promising target.42 Targeting this enzyme using selective inhibitors irreversibly damaged the assembly of inner membrane complex thereby impairing parasite replication both in vitro and in vivo. Another emerging candidate target is plasmodial signal peptide peptidase, a protease component of ER-associated degradation (ERAD)43 pathway, required for the degradation of unstable proteins.44 Although research pertaining to these novel targets is still evolving, these recent trends highlight the importance of chemical biology approaches in novel target hunting.
The development and design of new antimalarial drugs that are able to prevent invasion, rupture and other essential parasitic pathways will provide unprecedented advantage to combat the deadly infection of malaria. To facilitate the design of novel and efficient antimalarial drugs, it is important to understand parasitic genetics and resistance mechanisms in greater depth and resolution. Where employing classical genetic approaches has practical limitations in studying malaria, chemical biology combined to proteomics and biochemistry provide a suitable platform to probe hitherto unexplored molecular mechanisms and protein targets with great precision that can be exploited for future antimalarial design.
The authors acknowledge the following grants: SRG LSC 2013 049 (RC) and ZJURP1300102 (RC, AS and CTTC) by Singapore University of Technology. GS acknowledges President’s Graduate Fellowship awarded by the Ministry of Education, Singapore.
The author declares no conflict of interest.

©2014 Subramanian, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.