MOJ
eISSN: 2374-6920


L-asparaginase from fungal source finds extensive applications in pharmaceutical and food industries. L-asparaginase is an enzyme that catalyze the hydrolytic cleavage of the amino acid L-asparagine to L-aspartic acid and ammonia. In the present study, L-asparaginase was purified and characterized from the fungus, Fusarium culmorum ASP-87, isolated from tropical soil. The enzyme was purified to homogeneity by ammonium sulfate precipitation, ion exchange followed by gel filtration chromatography, to 14.03 fold with a final specific activity of 16.66U/mg of protein with 2.6% yield recovery. The molecular mass of the enzyme was 90kDa. The purified L-asparaginase had a pH optimum of 8.0 and a temperature optimum of 40°C. The enzyme was stable at pH 8.0 and retained 100% activity up to 24hrs, and at temperature 60°C retained 50% activity for 60min. The purified enzyme was highly specific to the substrate L-asparagine and the Km and Vmax were found to be 3.57mM and 0.5μmol/ml/min, respectively. The enzyme was activated by Mn2+ and Tween 80 and inhibited by Cu2+ and EDTA.
Keywords: fusarium culmorum asp-87, l-asparaginase, soil fungi, ion exchange chromatography, gel filtration chromatography
Microbial L-asparaginase is one of the most important industrial enzymes of interest accounting for about 40 % of the total worldwide enzyme sales.1 The enzyme L-asparagine amido hydrolase E.C.3.5.1.1 belongs to an amidase group that catalyzes the hydrolysis of the amino acid L-asparagine to L-aspartic acid and ammonia. This enzyme has got much significance in medical field for the treatment of leukemia especially acute lymphoblastic leukemia (ALL)2 and also widely used in baking and food industries to reduce the formation of carcinogenic acrylamides in biscuits and in deep fried potato.3,4 L-asparaginase is widely distributed in plants, animals and microorganisms. However, L-asparaginase from microbial sources has gained much attention because of its high productivity. It is extracellular and therefore secreted in to the fermentation medium. Among microbes, this enzyme is produced by bacteria, fungi and actinomycetes. Microbial strains like Escherichia coli,5 Erwinia caratovora,6 Pseudomonas aeruginosa,7 Streptomyces gulbargensis,8 Aspergillus terreus ,9 Aspergillus niger ,10 Penicillium brevicompactum,1 Cladosporium sp11 are the main source of L-asparaginase. Bacterial L-asparaginase has been reported to cause hypersensitivity leading to allergic reactions and anaphylaxis.12 Hence, L-asparaginase from eukaryotic microorganisms is gaining much importance as it is known to have less adverse effects.13 The main objective of the present work was to purify and characterize L-asparaginase from Fusarium culmorum ASP-87 isolated from tropical soil and to demonstrate its physio-chemical properties which are indispensible in both fundamental and applied research.
Strain and chemicals
The fungus F. culmorum strain ASP-87 used in this experiment was isolated from tropical soil. Media components used in the experiment were obtained from Hi-media (Mumbai, India). The substrate L-asparagine, DEAE-cellulose and Sephadex G-100 was procured from Sigma (Sigma-Aldrich, USA). All the chemicals were of analytical reagent grade.
Production of L-asparaginase by Fusarium culmorum ASP-87
The fungal culture was maintained on potato dextrose agar (PDA) slant at 4°C and sub cultured on PDA plates, incubated at 30°C for 6days and used as inoculum. The culture medium used for the study was modified Czapek-dox medium containing g/l of, Glucose,2.0; L-asparagine,10.0; KH2PO4,1.52; KCL,0.52; MgSO4.7H2O,0.52; CuNO3.3H2O,trace; ZnSO4.7H20,trace; FeSO4.7H20,trace; pH 7.5.14 Modified Czapek-dox broth (200 ml) was prepared, sterilized and inoculated with F. culmorum ASP-87 spore suspension (106/ml). The cultures were incubated at 30°C under shaken condition (120rpm) for 4days.
Assay of L-asparaginase activity
The activity of L-asparaginase in the culture filtrate was assayed using the method of Imada.15 The rate of hydrolysis of L-asparagine was determined by measuring the ammonia released using Nessler’s reagent. A mixture of 0.5ml of enzyme extract, 0.5ml of 0.04M L-asparagine, 0.5ml of 0.05M Tris-HCl buffer (pH 7.2) and 0.5ml of distilled water was incubated at 37°C for 30min. The reaction was stopped by the addition of 0.5ml of 1.5M trichloroacetic acid (TCA). The ammonia released in the supernatant was determined calorimetrically by adding 0.2ml of Nessler’s reagent into tubes containing 0.1ml of supernatant and 3.7ml of distilled water and incubated at room temperature for 20min. The absorbance was read at 450nm. One international unit (IU) of L-asparaginase activity is defined as the amount of enzyme required to produce 1µmol of ammonia per min under the conditions of the assay.
Purification of L-asparaginase
Ammonium sulfate precipitation: A four day old culture filtrate (200ml) of culmorum ASP-87 grown in modified Czapek-dox broth was collected after centrifugation at 8000rpm for 10min at 4°C. All subsequent purification steps were carried out at 4°C. The crude enzyme was subjected to ammonium sulfate precipitation and the protein precipitate at 80% salt saturation was allowed to stand overnight. The precipitate was collected by centrifugation at 10,000rpm for 15min and resuspended in 0.01M Tris-HCl buffer (pH-7.2) and dialyzed overnight against the same buffer.
Ion exchange chromatography: The dialyzed ammonium sulfate fraction was applied to an anion exchange chromatography on DEAE cellulose (1.5×15cm) column pre-equilibrated with the Tris-HCl buffer (0.01M; pH 7.2). The unbound proteins were washed repeatedly for five times and then eluted with the same buffer containing NaCl (1M) by a stepwise gradient at a flow rate of 1 ml/min. Fractions containing L-asparaginase activity were pooled and lyophilized.
Gel filtration chromatography: The lyophilized samples were loaded onto Sephadex G-100 (1.5×15cm) column pre-equilibrated with 100mM phosphate buffer pH 7.0 and eluted with Tris-HCl buffer (0.01M; pH 7.2). Fractions were collected (1ml/tube) and the absorbance of all the fractions was recorded at 280nm and active fractions were pooled and concentrated. The purified L-asparaginase was stored at 4°C and used for further characterization.
Estimation of protein: The concentration of protein was estimated by the method of Bradford16 using bovine serum albumin as the standard.
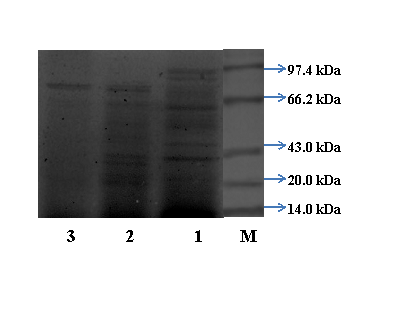
Determination of molecular weight of purified protein by SDS-PAGE: The molecular weight of purified L-asparaginase was determined by (SDS-PAGE) the method of Laemmli.17 SDS-PAGE was performed using a 12% polyacrylamide gel. The proteins were stained with coomassie brilliant blue R-250. The molecular weight of purified enzyme was determined using standard molecular weight markers (Bio-Rad).
Enzyme characterization
Effect of pH on enzyme activity and stability: In order to determine the effect of pH on purified L-asparaginase, the purified enzyme was pre-incubated in 0.1M buffer in the range between pH 3 and pH 11 without the addition of substrate, the enzyme activity was determined under standard assay conditions. Buffers used were sodium phosphate-citrate (pH 3.0-6.0), tris-HCl (pH 7.0-9.0) and glycine-NaOH (pH 10.0-11.0). In order to determine the pH stability, the purified enzyme was pre-incubated in pH buffer range between pH 3 and pH 11 at room temperature for 24hrs. The residual activity was determined for every 12hrs intervals under standard assay conditions. The activity of the enzyme at zero minute of the reaction was considered as 100% and served as control.
Effect of temperature on enzyme activity and stability: In order to determine the effect of temperature on purified L-asparaginase, the enzyme activity was studied at different temperatures ranging from 20°C to 80°C at 5°C increments, the enzyme activity was determined under standard assay conditions. Thermal stability of the purified enzyme was evaluated by incubating the enzyme for 10, 20, 30, 40, 50, 60minutes at different temperatures ranging from 30°C to 80°C. The residual activity was determined under standard assay conditions. The activity of the enzyme at zero minute of the reaction was considered as 100% and served as control.
Effect of metal ions, inhibitors and surfactants on L-asparaginase activity: In order to determine the effect of metal ions, inhibitors and surfactants like Mn2+, Zn2+, Fe2+, Cu2+, Mg2+, Ca2+, Hg2+, Co2+, Fe3+, EDTA, β-mercaptoethanol, tween 80 and SDS on L-asparaginase activity, the purified enzyme was preincubated with different metal solutions at 5mM concentration and inhibitors and surfactants at 1mM concentration for 30min and the residual activity of the enzyme was assessed. The activity of the enzyme without the addition of metal ions or inhibitors or surfactants was considered as 100% and served as control.
Substrate specificity
To assess the activity of L-asparaginase, different substrates such as L-asparagine, L-aspartic acid, L-glutamine and L-glutamic acid were used. The substrates were prepared in 0.05M tris- HCl buffer pH-7.5 at 10mM concentration. The enzyme was mixed with substrate and the reaction mixture was incubated for 30min at 40°C and enzyme activity was determined. Results were expressed as relative percentage.
Determination of kinetic constants
The kinetic parameters Km and Vmax of purified L-asparaginase were determined by the method of Lineweaver and Burk18 with different concentrations of L-asparagine (1Mm-10mM) dissolved in 0.05M of tris-HCl buffer pH 8.0. The enzyme activity was determined by measuring the rate of hydrolysis of L-asparagine under standard assay conditions.
Purification of L-asparaginase
The enzyme L-asparaginase was purified from the culture filtrate of F. culmorum ASP-87 using ammonium sulfate precipitation, ion exchange chromatography followed by gel filtration. The purification procedure is summarized in (Table 1). The first step of purification by ammonium sulfate precipitation achieved 2.63-fold purification with 26% enzyme recovery. The second purification step was done by ion exchange chromatography using DEAE cellulose. This step showed 8.42-fold increase in enzyme activity with 6.57% enzyme recovery. The final step of purification was performed by gel filtration using Sephadex G-100 column. The fractions showing L-asparaginase activity in this step were collected and pooled. The final step of purification resulted in 14.03-fold increase in enzyme activity with overall specific activity of 16.66U/mg of protein and with a net yield of 2.6% enzyme recovery. L-asparaginase from various fungal species have been purified and characterized and reported earlier. L-asparaginase from Penicillium brevicompactum NRC 829 was purified to 151.12 fold with a specific activity of 574.24U/mg and yield of 39.90%1 and L-asparaginase purified from Mucor hiemalis exhibited a specific activity 69U/mg with 18.46% recovery and 4.59 purification fold.19 Interestingly, L-asparaginase from Cladosporium sp was purified to 867.7 fold with a specific activity of 83.8U/mg.11 Although, the purification steps followed by various researchers are almost similar for different fungal species, the purification fold and yield varies. This could be due to the interference of different proteins present in the culture filtrate.
Sample |
Total Enzyme Activity (U) |
Total Protein |
Specific Activity (U/Mg) |
Purification Fold |
Yield (%) |
Crude |
95 |
80 |
1.1875 |
0 |
100 |
Ammonium sulfate precipitation |
25 |
8 |
3.125 |
2.6315 |
26 |
Ion exchange |
6.25 |
0.625 |
10 |
8.421 |
6.578 |
Gel filtration |
2.5 |
0.15 |
16.666 |
14.034 |
2.631 |
Table 1 Purification of L-asparaginase from F. culmorum ASP-87
Metal Ions (5mm) |
Relative Activity (%) |
Control |
100 |
Zn2+ |
77 |
Fe2+ |
68 |
Cu2+ |
16 |
Mg2+ |
93 |
Ca2+ |
96 |
Mn2+ |
118 |
Hg2+ |
20 |
Co2+ |
72 |
Fe3+ |
59 |
EDTA |
12 |
β-mercaptoethanol |
76 |
Tween 80 |
116 |
SDS |
0 |
Table 2 Effect of metal ions, inhibitors and surfactants on L-asparaginase activity
Physio-chemical characterization of the enzyme L-asparaginase
Determination of Molecular weight: The purified L-asparaginase from culmorum ASP-87 showed homogeneity and the molecular mass was estimated as 90kDa, by SDS-PAGE analysis (Figure 1). The molecular weight of L-asparaginase from F. culmorum ASP-87 was almost similar to that of Penicillium brevicompactum NRC 829 (94kDa) and Trichoderma viride (99kDa).1,20 Whereas, L-asparaginase from Cladosporium sp has a molecular weight of 117kDa and Aspergillus niger with 48kDa.13,21 The variability in the molecular weight of L-asparaginase in different organisms may be inferable to its genetic diversities.
Effect of pH on enzyme activity and stability: pH is a critical factor for stability and activity of purified enzyme, as it impacts on the ionic form of the enzyme active site residues. The effect of pH on the activity of purified L-asparaginase was done over a wide range of pH from 3.0 to 11.0 at 30°C. The results revealed that L-asparaginase from culmorum ASP-87 was active over a broad range of pH, optimum being pH 8.0 (Figure 2). Comparable results were reported by earlier workers in Penicillium brevicompactum NRC 8291 and Streptomyces sp.22 But, on the contrary Lincoln and Monica reported that pH 7.0 was the optimum pH for the activity of L-asparaginase in Trichoderma viride and Mucor hiemalis.19,20 The purified L-asparaginase retained 100% activity at pH 8.0 up to 24hrs of incubation (Figure 3). However, L-asparaginase from Trichoderma viride retained 82% of its activity after 24hrs of incubation20 and Mucor hiemalis maintained its stability only for 4hrs there after started declining.19 The stability of L-asparaginase from F. culmorum ASP-87 at alkaline pH is a promising factor to be used as a therapeutic agent.
Effect of temperature on enzyme activity and stability: Temperature is an important physical parameter which influences the enzyme activity. Effect of temperature on purified L-asparaginase was studied over a broad range of temperature from 30°C to 80°C. The optimum temperature for L-asparaginase activity was 40°C (Figure 4). However, further increase in temperature, declined the reaction rate sharply. Similar such results were reported by earlier workers in Aspergillus nidulans23 and Streptomyces griseoluteus.24 But, on the contrary Lincoln, Monica, Elshafi and Abraham reported that 37°C was the optimum temperature for the activity of L-asparaginase in Trichoderma viride, Mucor hiemalis, Penicillium brevicompactum NRC 829 and Fusarium1,19,20,25 The thermal stability studies of purified L-asparaginase revealed that the enzyme was highly stable for 120min at 30°Cto 40°C (Figure 5), but however, inactivated at temperature above 40°C while retaining 50% activity at 60°C for 1hour. L-asparaginase from Penicillium brevicompactumNRC 829 was reported to be stable up to 1hour at 37°C.1 Considering the thermal stability of other organisms reported earlier, L-asparaginase from F. culmorum ASP-87 shows moderate thermostability, a factor of significant importance in pharmaceutical industry.
Effect of metal ions, inhibitors and surfactants on L-asparaginase activity: The influence of various metal ions on purified L-asparaginase activity was studied. Among the different metal ions tested Mn2+ enhanced the activity of enzyme by 18% (Table 2) as reported by earlier worker in Mucor hiemalis.19 Whereas, Cu2+ and Hg2+ inhibited the activity of enzyme by 84 and 80%, as observed by Archana in Aspergillus nidulans23 and Kumar in Pectobacterium carotovorum.26 However, other metal ions like Ca2+ and Mg2+ did not have much effect on enzyme activity. Among the two inhibitors tested, EDTA inhibited the activity by 88% while β-mercaptoethanol did not have any effect on enzyme activity.20 However, Senthil kumar & Selvam27 reported that EDTA act as an inducer in Streptomyces radiopugnans 27 Among the surfactants tested, the non ionic surfactant tween 80 was found to enhance the activity of the enzyme by 16%, whereas, the anionic surfactant SDS completely inhibited the enzyme activity. Kumar and Monica also reported similar such finding that tween 80 at 2mM concentration induced the production of L-asparaginase in Cladosporium sp and Mucor hiemalis.11,19
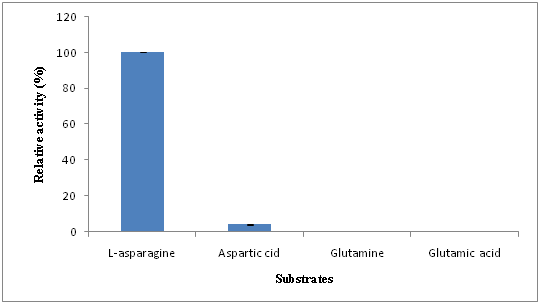
Substrate specificity
The property of enzymes that makes them important as diagnostic tools is the specificity they exhibit towards the substrate. Among the different substrates investigated, the enzyme showed high specificity towards its natural substrate L-asparagine, very low specificity towards L-aspartic acid, while no activity towards L-glutamine and L-glutamic acid (Figure 6). Our findings were in concordance with that of Prabhu, Sahira and Lincoln in Vibrio costicola, Acinetobacter baumannii and Trichoderma viride respectively.20,28,29 This property of purified enzyme increases its potential to be used in therapeutics and food industries.
Determination of kinetic constants
The Km and Vmax of purified L-asparaginase from F. culmorum ASP-87 were determined using various concentrations of L-asparagine. The values of Km and Vmax were 3.1mM and 0.77μmol/ml/min respectively (Figure 7) which is lower than the Km value of L-asparaginase purified from E. coli, Penicillium sp and Mucor hiemalis.19,30,31 However, a slightly higher Km value of 12.5mM and lower Km value of 1.05mM were reported in Aspergillus aculeatus and Penicillium brevicompactum.1,32 The purified L-asparaginase from Fusarium culmorum ASP-87 has stronger affinity towards its natural substrate L-asparagine, a positive property to be useful towards the treatment of tumors.
L-asparaginase from microbial source is of great interest owing to its significance in pharmaceutical and food industries. Therefore, in the present study, an attempt is made to purify L-asparaginase from Fusarium culmorum ASP-87 and its physical and chemical characteristics were studied. The stability of L-asparaginase at alkaline pH and temperature is an advantage for pharmaceutical and food applications. The specificity of L-asparaginase to its natural substrate L-asparagine is of greater importance for making diagnostic biosensors. However, further in depth studies are required to optimize cost effective substrate for bulk production of enzymes. Although, a strain of Fusarium culmorum IFO5902 is been reported to possess L-asparaginase activity by Nakahama way back in 1973,33 no attempt was made so far to purify and characterize the physio-chemical nature of the enzyme. This is the first report which gives the complete physical and chemical nature of the purified L-asparaginase from Fusarium culmorum, isolated and identified as one of the best producers of L-asparaginase by dye based rapid screening method among the 364 soil fungal isolates screened.34
The author acknowledges Department of Microbiology and Biotechnology, Bangalore University, Bangalore, India, for their kind support.
The author declares no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.