MOJ
eISSN: 2374-6920


The physical nature of mechanisms by which cells sense radiation-induced DNA-strand breaks remains to be elucidated. The fast induction of, for example, ATM kinase activity immediately after exposure to ionizing radiation, suggests that it acts at an early stage of signal transduction. Existing data indicate that ATM activation is not dependent on direct binding to strand breaks. However, some fundamental questions are still not responded to, such as: how ATM directly senses structure disruption in “relaxed” chromatin, and which factors determine the impressive speed and extent of the ATM response. Here, a biophysical model for the signaling mechanism of both the instant activation of repair proteins, and the recognition of a few DNA breaks by these proteins within the entire genome is proposed. The model allows for the calculation of an electromagnetic transient, produced by an electron coherent hopping current generated by the DNA damage, which is able to induce long-range activation (phosphorylation) of repair proteins almost instantly. Existing experimental evidence verifying this approach is discussed. The possible role of this study in stimulating and orientating novel applications in medical physics, as e.g. radiotherapy, is, furthermore, addressed.
Keywords: DNA radiation damage, DNA damage sensing, DNA repair, quantum-coherence hypothesis, radiation sensitizer, radiotherapy, phosphorylation, radiotherapy, ATM kinase, apoptosis, biochemical signal, static electric field, mutagenesis, chemotherapies, mammalian cells
DNA repair machinery – the role played by the ATM kinase
Ionizing radiation is by far the most deleterious agent to our genetic material, since it is able to induce double-strand breaks (DSB) in DNA. In this sense, cells have evolved complex signaling pathways to arrest the progression of the cell cycle in the presence of DNA damage, thereby providing increased time for repair mechanisms to operate. Finally, when the burden of genomic insult is too large to be effectively met by the various cell responses, cells are able to initiate programmed cell death (apoptosis).1 It is known that the protein kinase ATM is the central player in mammalian cell response to ionizing radiation, playing an important role in the activation of cell cycle checkpoints that lead to DNA damage-induce arrest at G1/S, S, and G2/M. Following ATM activation, phosphate groups are added (phosphorylation) to several target proteins, p53 in particular. Several reviews have dealt with many properties of ATM – its autophosphorylation is an important upstream event in the activation pathway. However, it is still a debated issue whether ATM, located outside the cell nucleus, is the primary sensor of DNA damage.2–10 Over a decade ago, in this regard,4 showed that in the normal, undamaged cell the ATM proteins are tightly linked in pairs, the dormant ATM dimmers. Following DSB formation, autophosphorylation of ATM is induced, thus separating the two ATM proteins. The authors concluded that activation of the ATM kinase appears to be an initiating event in cellular responses to irradiation toward the subsequent repair process. Additionally, these authors concluded that DNA breaks must somehow signal to ATM molecules at a distance in the cell and that, moreover, direct binding of a protein complex to DNA breaks (resulting in ATM activation) is rather unlikely. Finally, it was proposed that the introduction of DSBs causes a rapid change in some aspects of higher-order chromatin structure and that consequently this chromatin alteration would initiate ATM activation.
DSB formation versus ATM activation – unanswered questions
Jakob and collaborators (2005) performed a sophisticate experiment at the Darmstadt/Germany accelerating facility, where mammalian cells were irradiated with heavy-ion beams, aiming at the study of protein recruitment dynamics to DNA lesions. Thus, using heavy-ion irradiation to generate localized damage in the nucleus, and with the aid of immunocytochemical microscopy detection (on GFP-tagged DNA repair proteins), it was possible to observe protein translocations to sites of ion traversals. Two key conclusions of this work are:
Interesting too, the data analysis revealed only a small radiation-independent overall movement of chromatin. This small non-directional migration of chromatin appeared to correspond to a constrained Brownian motion.11 On the other hand, the very fast responses after generation of DNA damage show that protein translocations, consistent with DNA damage recognition, occur almost immediately. These results are at variance with propositions that the activation of signal cascades and the processing of lesions take place following DNA damage-induced rearrangements of chromatin structures. As pointed out elsewhere,7 chromatin remodeling occurs frequently as genes are expressed, and during every cell-division cycle when DNA is replicated, yet ATM seems to ignore such alterations. Therefore, a plethora of intriguing, key, and first principles questions, raised by all of these studies, still remain unanswered. It is here suggested that they can be grouped into 3 topics: signaling/sensing, navigation/recognition and timing/extent.
Signaling/Sensing
Navigation/Recognition
Timing/Extent
It is here described how an electromagnetic field would be produced at a DNA damage site, thus providing “cause-effect” answers to all the questions formulated above. This approach comprises the following sequence of events:
Formation of an electromagnetic transient around the DSB site
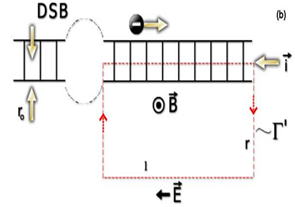
The phosphates in the DNA backbone make DNA one of the most highly charged polymers known. It has linear charge density of one fundamental charge (negative) per 0.17 nm of its length.12 Electron transfer and transport along DNA are likely to play an important role in radiation damage and repair. As pointed out elsewhere (Yu and Song, 2001) , a DNA double helix with a random base pair sequence can be viewed as an 1D disordered system. In this system, the disorder leads to electronic localization, and electron hopping between these localized impurity states along the chain are responsible for the conductivity. Electron-transfer reactions can occur under such conditions via two principal mechanisms.13
The first process is quantum-coherent, in the sense that the electron does not exchange any energy with the molecule during the transfer. Additionally, for short-distance motion (over a few base pairs), coherent tunneling can occur but exhibits an exponential decay as a function of the traveled distance (b). In fact, an exponential decay of the conductance (C) with increasing distance (b) has been observed directly in DNA molecules folded into hairpin shapes (Figure 1).14 Therefore, an equally strongly varying electric current is established. Well-known in Electrodynamics, an electric current generates a magnetic field, and that if this field changes with time an electric field is also induced (Figure 1). In the particular case of an electric current in damaged DNA, as depicted above, a short duration electric pulse (a transient) is produced, as shown in Figure 2. Detailed calculations are presented in the Appendix 1.
The underlying static electric field at the damaged site
DSBs also generate static electric and magnetic fields, as revealed by experiments of perturbed angular correlations of gamma rays (PAC), an alternative and elegant experimental technique to study the molecular dynamics of DNA. Quite long ago, experimental results suggested that the static electric fields are quadrupole fields.15,16 While electric transients have short duration, static electric fields persist until completion of repair, also contributing to the signaling and orientation of the repairing proteins, as di scussed below.
How are the ‘dormant’ dimmers “awakened”? The specificity and rapidity issues
The ubiquity of an electric transient all over the cell volume explains much of the peculiarities observed in the repair process of radiation induced DSB. Actually, the post- DSB formation scenario, as depicted by the present approach, is the one where all points of the cell interior are experiencing an electromagnetic transient, with an intensity decreasing monotonically with the distance from the damage site, and with time duration of the order of some “biological times” (Appendix 1). It is like a flash illuminating the cellular interior. The electromagnetic nature of the signal generated by the DSB explains the rapidity of the ATM activation process, as experimentally indicated by the very fast recruitment to damage sites.11 A fundamental aspect remains to be elucidated: specificity. However, this is not a serious issue in electrodynamics, since it is certainly associated with the peculiarities of signal receiving processes, as in a radio antenna, where only those electromagnetic wave packets in resonance with the antenna receiving modes are tuned up. This insight provides a raison d’etre for the ubiquitous prevalence of helical structures in biology, which would serve as antennas for electric pulses generated at the DNA damage sites, and also generating pulses of acceleration energy on charges (mostly electrons) traversing the helices.17 Therefore, those damages generating electric pulses, capable of resonating within the helical structures of certain proteins, would trigger the repair processes conducted by these proteins, where phosphorylation is prevalent in the earlier stages. It is noted, in this regard, that the “biochemical signal” necessary to start hydrolyzation of an ATP molecule is, highly probable, an electric transient as in muscle contraction, which is triggered by an action potential at the site where a nerve impinges on the muscle-cell membrane. This quick electrical discharge releases Ca2+ ions.18,19 Under these circumstances, an electric transient activates locked ATM’s following DNA damage.
Navigation/Recognition
Another unanswered key question, already mentioned above, is “how a few DSBs within three billion base pairs are recognized by the repairing enzymes (e.g. ATM and/or its substrates)”.7 The most plausible answer is the inference that the gyrocompass assuring the correct navigation route is the alignment of the ATM electric dipole (which is rather huge in proteins) along with the underlying static electric field at the damage site (working as a navigation cue). Thus, the static electric field at the DSB site works as an instantaneous “marker”. Such an electric sensing possibility could be at the heart of the explanation provided a decade ago by Banerjee et al.,20 on how the human repair enzyme 8-oxoguanine glycosylase finds aberrant nucleotides (from oxidative damage) among the myriad of normal ones. Actually, the opening and closing of voltage-activated Na+, Ca2+ and K+ (Kv) channels underlies electrical and chemical signaling throughout biology, yet the structural basis of voltage sensing is unknown. Because of, for example, the presence of salt ions in the cytoplasm, one would argue that the net charge at each DSB site is gradually screened during the signaling-repairing time interval. However, although undergoing reorientation and attraction by the electric field toward the DSB site, ion movement is not propelled as in the repair protein; therefore, while the former moves diffusively, an ATM monomer, for example, moves processively.21
Below, existing experimental data supporting the biophysical model here proposed (see section 2 – Goals) is discussed.
Navigation/recognition
As addressed above (paragraph 3.2), the underlying static electric field at the DSB site assures the correct navigation route. This is accomplished by means of the alignment of the ATM electric dipole with the static electric field at the DSB site (hypothesis at paragraph 2.2). A series of experiments was carried out at this Laboratory with prokaryotes and eukaryotes exposed to ionizing radiation and exogenous static electric fields.22–25 All results show that when cells exposed to radiation are immediately submitted to static electric fields, cell death increases enormously compared to the effect of radiation alone. Since the sole application of an electric (E) field has no effect on control samples, it was thus demonstrated that the E-field acts on the DNA repair process. Once the only possible effect of a static electric field is polarization, one is led to infer that repair proteins gradually tend to align their electric dipoles with the direction of the E-field. Consequently, the majority of these proteins would be unable to reach damaged sites in DNA, and lack of repair, particularly of DSB, may lead to cell death. However, a much more stringent support to this possibility was provided by measurements of γ-H2AX foci. By the action of a static electric field on the irradiated MRC5 cells (strain of lung cells) the number of nuclei with γ-H2AX foci increased 40%. These results indicate that static electric fields directly interfere in DNA repair mechanisms by inactivating cellular DNA repair processes, as revealed by the 40% surplus of nuclei with γ-H2AX foci in MRC5 cells.22
Highly revealing too, Moron et al.,25 at this Laboratory, irradiated T47D breast cancer cells with 1 and 2Gy of gamma radiation, and then exposed them to a static electric field of 1250V/cm by 24hours. From cell cycle distributions evaluation by flow cytometry, it was found that treatment with irradiation and a static electric field (SEF) exposure causes a higher accumulation of cells at the S phase, and a corresponding reduction at G1, while the population in G2/M remained nearly unchanged. It was concluded that a SEF interferes with the progression trough S-phase in irradiated cells, most likely due to an interference with DNA repair mechanisms, thus working as a radiation sensitizer for possible clinical application. It is clear that there is no possibility for a direct observation of an endogenous electric field (at a DSB site) interacting with a repairing enzyme (e.g. the ATM, in the case of mammalian cells). However, the results obtained at this Laboratory strongly indicate that, although exogenous, E-fields interfere with repairing enzymes. Therefore, the reverse process should be true, that is, through electric dipole orientation repair proteins sense the static electric field at the damage site and use it as a navigation cue. Interestingly, such a possibility complements the explanation provided by Banerjee et al.,20 on how the human repair enzyme 8-oxoguanine glycosylase finds aberrant nucleotides (from oxidative damage) among the myriad of normal ones.
Signaling/sensing
Biological effects at the cellular level induced by electromagnetic radiation, as e.g. phosphorylation, are well established. Fairly interesting in this regard, over three decades ago Goodman et al.,26 demonstrated that very weakly pulsing exogenous electromagnetic fields (pulse amplitude around 0.15V/m) induce transcription of genes in cultured salivary gland cells of the fruitfly. In fact, weak direct currents have modified basic cellular phenomena such as growth, differentiation, dedifferentiation, and repair. Weisbrot et al.,27 however, found out that low-frequency exposures on Drosophila melanogaster, during the 10-day developmental period from egg lying through pupation, induced the phosphorylation of the nuclear transcription factor ELK-1.
Therefore, the hypothesis worked out here makes strong sense, stating that the phosphorylation of head-proteins in cascade repairing reactions, as e.g. ATM in mammalian cells, MEC1 in S. cerevisiae and RAD3 in S. pombe, is induced by an electromagnetic (EM) transient emitted from the DNA damaged site.23 While providing a conceptually correct cause-effect relationship for the primary DNA signaling process, this hypothesis points to the very likely possibility that EM fields initiate phosphorylation by interacting directly with moving electrons in proteins. This is precisely the analog of electromagnetic initiation of DNA transcription.28
As pointed out elsewhere, there is a research field on biological responsiveness to DNA damage, encompassing and integrating aspects as diverse as DNA repair, mutagenesis, programmed cell death, and damage tolerance among others.29 The development of new strategies for blocking the unwanted consequences of DNA damage, cancer in particular, is envisaged. If cancer were already installed, however, the insights presented here would facilitate development of approaches to make tumors more sensitive to radiation and chemotherapies. In fact, the main motivation of studies on the molecular basis of cancer is to develop new therapies for tumors where there is no effective treatment, or where existing therapies have significant side effects or problems. Radiation therapy, for instance, is now quite promising with the advent of hospital-based heavy ions accelerators, providing a therapy that minimizes the killing off of normal cells. However, existing therapies could be greatly enhanced if radiation doses are kept to a minimum, and their tumor cell killing efficiency is kept to a maximum. Therefore, the combination of radiation with simultaneous application of static electric fields (SEFs) in the tumor area could work in this direction.25 This biological role of SEFs as radiation sensitizers is a relevant issue, since stand-alone exposure to this exogenous physical agent is not genotoxic.
The limits of this radio sensitization process induced by SEFs were tested by carrying out viability measurements with highly radioresistant cell lines, such as Deinococcus radiodurans, one of the fiercest radioresistant organisms, exhibiting a sophisticated repair mechanism responsible for its extremophilic character.22,24 It should be noted that the present approach, using long-range electron transfer and conduction in DNA (Appendix 1) is high on the agenda of many biochemistry and biophysics research groups, as evidenced by a Special Issue section in PNAS-102-see, in particular.30–32 The hypothesis here developed, by constituting a reasonable guideline for more elaborated signaling/transduction models, is also an original and effective contribution to the research field on cellular responses to genomic insult. Therefore, the present biophysical approach points to the possible existence of another link between DNA damage, changes in chromatin structure, and downstream signaling events, which could stimulate and orientate novel studies and applications in this area.33–35
This work was partially supported by grants from CAPES and CNPq, Brazilian Foundations.
The author declares no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.