MOJ
eISSN: 2574-8130


Research Article Volume 3 Issue 1
1Chita State Medical Academy, Russia
2Innovative Clinic Health Academy, Russia
3Medicine C Department, Clinical Immunology and Allergy Division, Ben Gurion University of the Negev, Israel
Correspondence: Eli Magen MD, Medicine C Department, Clinical Immunology and Allergy Division, Ben Gurion University of Negev, Zionut 21/33, Ashdod, 77456, Israel
Received: December 01, 2017 | Published: February 9, 2018
Citation: Kuznik BI, Davydov SO, Stepanov AV, et al. The role of growth differentiation factors 11 and 15 (GDF11, GDF15), eotaxin-1 (CCL11) and junctional adhesion molecule a (JAM-A) in the regulation of blood pressure in women with essential hypertension. MOJ Gerontol Ger. 2018;3(1):71-75. DOI: 10.15406/mojgg.2018.03.00089
Introduction: Evaluation of biomarkers of arterial hypertension (AH) could be considered to be useful at early stages of hypertension.
Objective: We hypothesized that the levels of junctional adhesion molecule-A (JAM-A), eotaxin/C-C motif chemokine ligand 11 (CCL11), growth differentiation factor 11 (GDF11), and growth differentiation factor-15 (GDF15) might be a biochemical markers of AH.
Methods: We examined the association between blood levels of these chemokines and blood pressure in middle-aged female hypertensives in three groups; 37 patients treated by antihypertensive medications (T-AH) group, 16 untreated hypertensive patients (U-AH group) and 29 healthy subjects (control group). JAM-A, CCL11, GDF11, and GDF15 concentrations were evaluated with ELISA.
Results: The patients of T-AH group and UT-AH group were characterized by higher blood levels of GDF15 (13.9±0.9 pg/ml - T-AH group and 14.6±5.9 pg/ml - UT-AH group) than normotensive control subjects (6.1±2.3 pg/dl; p<0.001). Blood levels of JAM-A was also higher in T-AH group (3.5±0.4 ng/ml) and UT-AH group (3.3±0.9 ng/ml), than in Control group (2.8±0.6 ng/ml). The higher blood CCL11 levels were found in T-AH group (260.9±32.3 pg/ml), than in UT-AH (223.5±48.4 pg/ml; p=0.0017) and Control groups (187.7±36.8 pg/ml; p<0.001). Levels of GDF11 were significantly lower in both treated (9.3±1.1 pg/ml) and untreated hypertensives (9.2±3.4 pg/ml), than in normotensive control subjects (31.3±8.6 pg/ml, p<0.001.)
Conclusion: Blood CCL11 and GDF15 are elevated and GDF11 is decreased in human hypertension. There is a negative correlation between blood GDF15 levels are negatively correlated with systolic blood pressure in patients with treated AH.
Keywords: Jam-A, ccl11, gdf11, gdf15, arterial, hypertension
Evaluation of biomarkers of arterial hypertension (AH) could be considered to be useful as early, more reliable and accurate diagnostic indicators for AH than simply measuring of blood pressure (BP) in routine clinical practice, especially at early stages of hypertension. For instance, an increased level of a soluble protein junctional adhesion molecule-A (JAM-A) was previously found to be associated with human hypertension.1,2 JAM-A upregulation may be triggered by AT1 receptor-mediated signaling and therefore it is suggested that JAM-A may have a prognostic and possibly a pathogenic role in the development of AH.3 Recently, Bhat SA et al.4 found that in patients with AH the expression of adhesion molecules (including JAM-A) on brain endothelium is increased.4
Recent studies suggest that the eotaxin/C-C motif chemokine ligand 11 (CCL11) may also participate in the pathophysiology of cardiovascular diseases.5 Currently, plasma CCL11 levels have been considered to be the age-associated biomarker and blockade of CCL11 can suppress aspects of age-related cellular dysfunction.6
In Loffredo at al.7 reported an age-dependent decline in circulating growth differentiation factor 11 (GDF11) protein levels in mice and it was demonstrated that restoring GDF11 to youthful levels reduces cardiac hypertrophy in older mice.7 In addition, the data from large human cohorts reveal that circulating GDF11 levels decline with age, and low GDF11 levels are associated with an increased risk of cardiovascular events.8 GDF11 is a member of the TGF β superfamily of proteins and it is the most closely related to myostatin a negative regulator of muscle mass.9 Growth differentiation factor-15 (GDF15) is another member of the TGF-β super family and it has also been suggested to be an important biomarker of cardiovascular pathology due to its regulatory roles in inflammatory and trophic responses during tissue injury.10
We hypothesized that the levels of JAM-A, CCL11, GDF11, and GDF15 might be biochemical markers of human hypertension. We therefore examined the association between systemic levels of these chemokines and arterial hypertension in middle-aged Russian female participants.
Patients
This prospective study examined 37 consecutive treated with antihypertensive medications female hypertensive patients from the Arterial hypertension Clinic (T-AH group) and 16 consecutive female never treated hypertensive age matched patients (UT-AH group) at Chita Medical Academy Hospital. The control group comprised 29 healthy normotensive volunteers matched for age and sex. All patients provided written informed consent, and institutional ethics committee at Chita Medical Academy approved the study protocol.
Arterial hypertension (AH) was diagnosed on the basis of results of 24h blood pressure measurement. ABPM was performed by using Mobil-O-Graph NG monitors (IEM, Stolberg, Germany). Monitors were programmed to obtain BP readings every 15 minutes from 07:00 to 22:00, and every 30 minutes from 22:00 to 07:00.
The values of blood pressure used in the study are mean of 3 consecutive measurements after 20 minute rest in sitting position performed at the day of blood drawing. AH was defined according to the European Society of Hypertension and European Society of Cardiology as blood pressure≥140/90 mmHg.11 The normotension criterion was blood pressure≤139/89 mmHg. All T-AH group patients were treated with hypotensive drugs for median period of 5.3±1.8 years (Table 1).
T-AH Group N=37 |
UT-AH Group N=16 |
Control Group N=29 |
Р |
|
Sex (M/F) |
0/37 |
0/16 |
0/29 |
|
Age (years) |
57.3±2.7 |
55.2±3.6 |
56.8±2.9 |
0.071 |
BMI (kg/m2) |
27.7±2.4 |
27.3±2.8 |
26.9±2.5 |
0.442 |
Waist circumference (cm) |
84.1±9.8 |
82.9±10.4 |
83.6±10.1 |
0.848 |
Fasting glucose (mg/dl) |
91.7±9.2 |
90.4±8.7 |
93.5±8.9 |
0.513 |
Total cholesterol (mg/dl) |
187.9±45.2 |
185.7±54.6 |
191.1±49.4 |
0.932 |
LDL cholesterol (mg/dl) |
125.3±37.1 |
120.9±42.3 |
123.7±42.9 |
0.935 |
HDL cholesterol (mg/dl) |
46.5±18.2 |
47.2±18.7 |
49.1±17.4 |
0.841 |
Fasting triglycerides (mg/dl) |
131.4±46.7 |
137.2±51.9 |
140.2±55.1 |
0.777 |
Systolic blood pressure (mmHg) |
135.7±11.5 |
162.4±9.3 |
124.5±8.5 |
< 0.001 |
Diastolic blood pressure (mmHg) |
79.4±9.3 |
85.8±5.1 |
75.2±6.7 |
< 0.001 |
Antihypertensive Therapy |
||||
ACE -I n (%) |
24 (64.9%) |
0 |
0 |
|
Diuretic n (%) |
21 (57.8 %) |
0 |
0 |
|
ARB n (%) |
10 (27 %) |
0 |
0 |
|
Β blocker n (%) |
9 (24.3 %) |
0 |
0 |
|
Ca-channel blocker n (%) |
11 (29.7 %) |
0 |
0 |
|
GDF15 (pg/ml) |
13.9±1.9 |
14.6±5.9 |
6.1±2.3 |
< 0.001 |
GDF11 (pg/ml) |
9.3±1.1 |
9.2±3.4 |
31.3±8.6 |
˃ 0.001 |
CCL11 (pg/ml) |
260.9±32.3 |
223.5±48.4 |
187.7±36.8 |
< 0.001 |
JAM-А (ng/ml)) |
3.5±0.4 |
3.3±0.9 |
2.8±0.6 |
< 0.001 |
Table 1 Characteristics of hypertensive patients and healthy controls.
T-AH group: Patents with Treated Arterial Hypertension; UT-AH Group: Patients with Never Treated Arterial Hypertension; BMI: Body Mass Index; LDL: Low-Density Lipoprotein Cholesterol; HDL: High-Density Lipoprotein Cholesterol; ACE-I: Angiotensin-Converting Enzyme Inhibitors; ARB: Angiotensin II Receptor Blockers; GDF11: Growth Differentiation Factor 11; GDF15: Growth Differentiation Factor-15; CCL11: Eotaxin/C-C Motif Chemokine Ligand 11; JAM-А: Junctional Adhesion Molecule-A.
Exclusion criteria were the following: secondary hypertension, diabetes, ischemic heart disease, heart failure, hormonal replacement therapy, existing or previous infections in the 6 weeks before the study, neoplastic and autoimmune diseases. Active smokers were also excluded from the study.
Sample preparation
Fasting blood samples were drawn from all the participants in sitting position from forearm vein and consequently were used to evaluate standard clinical parameters and serum concentration of JAM-A, CCL11, GDF11, and GDF15 (Table 1). Serum was obtained from blood samples after centrifugation at 500 xg for 15 minutes. Then serum was allotted into 0.5 ml aliquots, and stored at -80°C until analysis.
JAM-A, CCL11, GDF11, and GDF15 concentrations were evaluated with ELISA tests (Cloud-Clone Corp. Houston,, USA) according to the manufacturer instructions. The detection range for JAM-A was 0.312-20ng/mL, for CCL11 - 15.62-1000pg/mL, for GDF15 - 0.156-10ng/mL, and for GDF11 - 15.6-1000pg/mL.
Statistical analysis
Values are shown as mean ± standard deviation. The results were calculated with Statistica 10.0 software (StatSoft, Tulsa, Oklahoma, USA). The one-way analysis of variance (ANOVA) was used to determine differences among the three groups. Correlations between variables were analyzed by Spearman rank correlation. Values of p<0.05 were considered to be statistically significant.
Hypertension is associated with increased serum concentration of JAM-A, CCL11, GDF15 and with decreases serum concentration of GDF11
The patients with both treated (T-AH group) and untreated (UT-AH group) hypertension were characterized by higher blood levels of GDF15 (13.9±0.9 pg/ml – T-AH group and 14.6±5.9 pg/ml – UT-AH group) than normotensive control subjects (6.1±2.3 pg/dl; p<0.001). Serum levels of JAM-A was also higher in T-AH group (3.5±0.4 ng/ml) and UT-AH group (3.3±0.9 ng/ml), than in Control group (2.8±0.6 ng/ml). The higher serum CCL11 levels were found in T-AH group (260.9±32.3 pg/ml), than in UT-AH (223.5±48.4 pg/ml; p=0.0017) and Control groups (187.7±36.8 pg/ml; p<0.001).
On the contrary, blood levels of GDF11 were significantly lower in both treated (9.3±1.1 pg/ml) and untreated hypertensives (9.2±3.4 pg/ml), than in normotensive control subjects (31.3±8.6 pg/ml, p<0.001) (Table 1).
Correlations between serum concentration of JAM-A, CCL11, GDF15, GDF11 and blood pressure
In T-AH group blood levels of GDF15 were in negative correlation with systolic blood pressure (SBP)(r=-0.51, p=0.006) (Figure 1).
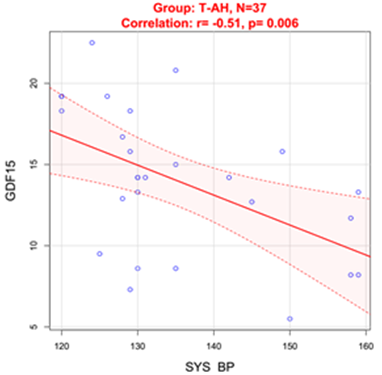
Figure 1 Correlation between SBP and GDF15 in treated hypertensive patients.
SYS BP: Systolic Blood Pressure
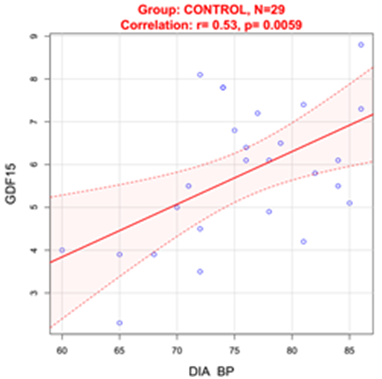
Figure 2 Correlation between DBP and GDF15 in normotensive Control group.
DIA BP: Diastolic Blood Pressure
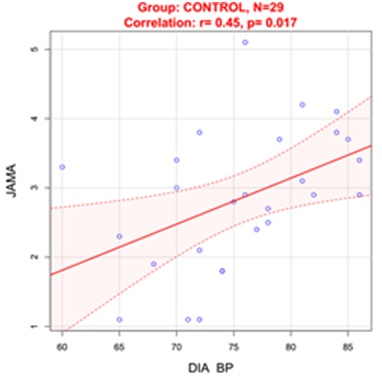
Figure 3 Correlation between DBP and JAM-A in normotensive Control group.
DIA BP: Diastolic Blood Pressure
In Control group diastolic blood pressure (DBP) was positively correlated with blood GDF15 levels(r=0.53, p=0.0059) (Figure 2) and serum JAM-A levels (r=0.45, p=0.017) (Figure 3).
There were no statistically significant correlations between serum concentrations of JAM-A, CCL11, GDF15, and GDF11 in all study groups.
This study evaluated four blood chemokines, JAM-A, CCL11, GDF11, and GDF15 in patients with AH. Our study included groups of treated and untreated hypertensive female patients and matched normotensive controls with similar demographic characteristics. As it is shown in Table 1, statistically significant differences were observed between the study groups in serum levels of GDF15, GDF11, CCL11 and JAM-А. Here, we report for the first time that the level of blood CCL11 is elevated and the level of GDF11 is decreased in human hypertension. Another finding of our study is that although blood GDF15 is elevated in the untreated and treated hypertensive subjects, there is a negative correlation between blood GDF15 levels and SBP in patients with treated AH. No statistically significant correlations were observed between serum concentrations of JAM-A, CCL11, GDF15, and GDF11 in all study groups.
As previous studies showed that GDF15 is age and sex related biomarker12 additionally, age and sex are critical variables in the expression of most inflammatory signals13 thus we selected only aged matched female subjects.
As in the previous study14 blood GDF-15 levels in hypertensive patients were found to be significantly higher than in healthy volunteers and were negatively correlated with SBP. Lately Hao Xue et al. found that blood GDF-15 levels were positively related to measures of left ventricular hypertrophy in hypertensive patients.15 GDF-15 is not expressed in cardiovascular system under normal physiological conditions but increases rapidly in response to pressure overload.16 Therefore, we suppose that the observed high blood GDF15 levels in T-AH and UT-AH groups may indicate a possible involvement of GDF15 in the pathophysiology of AH.16 We can also speculate, that the observed negative correlation between blood GDF15 levels and SBP in treated hypertensive patients may be explained by the influence of antihypertensive medications. Previously, irbesartan (AT1 receptor blocker) was found to significantly reduce angiotensin II induced GDF-15 expression in cardiomyocytes.15
GDF15 is expressed and secreted in response to inflammation, oxidative stress, hypoxia, telomere erosion, and oncogene activation and it has been recently recognized as a cardioprotective cytokine.17 Higher blood concentrations of GDF15 are associated with increased risks of developing cardiovascular disease, while low concentrations of GDF15 are closely associated with longevity.18 Moreover, previous studies demonstrated that genetic variants of GDF-15 are associated with cardiac remodeling in patients with AH.19 Recently, the same research group showed that elevated GDF-15 level is associated with the development of first-ever stroke in the patients with AH.20 GDF-15 is a stress-induced biomarker and in pressure overload its increase might be protective.21 We hypothesize that during blood pressure elevations an expression of GDF-15 can be increased and it is secreted into the blood to exert protective function.21 Another interesting finding of our study is the positive correlation between blood GDF15 levels and DBP in normotensive control group. This finding gives us reasons to theorize that GDF15 might play some role in physiology of blood pressure regulation. Further studies are needed to check this hypothesis and to examine whether blood GDF15 levels can serve as a biomarker of antihypertensive treatment efficacy in human hypertension.
The results of our study confirm the previous data that the level of blood JAM-A is elevated in AH, nevertheless we did not observe positive correlations of JAM-A with blood pressures as it was found in the previous study.22 While in the previous study JAM-A was elevated in hypertensive patients with untreated hypertension compared with normotensive patients and reduced in patients treated with renin-angiotensin system antagonists22 we did not find any difference in blood JAM-A levels between the patients with treated and untreated hypertension. Probably, the size of our study groups was too small to see statistically significant differences between T-AH and UT-AH groups, as well as to find significant correlations between JAM-A levels and blood pressure. Nevertheless, in our small normotensive control group DBP was positively correlated with blood JAM-A levels. This novel finding should be confirmed in larger cohorts in the further trials.
Another important finding of our study is that blood CCL11 concentrations were increased in hypertensive subjects. Moreover, blood CCL11 levels in the T-AH group with longstanding hypertension were higher than in newly diagnosed and never treated UT-AH group. CCL11 is mainly associated with allergy and this chemokine has been identified as a potential biomarker for the diagnosis and assessment of asthma, atopic dermatitis and inflammatory diseases of the bowel.23 Previously, the association between CCL11 polymorphisms with the prevalence of hypertension was shown in Japanese men in the population-based longitudinal cohort study24but there are no other studies on the role of CCL11 in AH. The possible role of CCL11 in pathophysiology of hypertension has to be elucidated in further basic and clinical studies. At this point we can only speculate that CCL11 could by some means regulate endothelial cell function and inflammation in AH.25 Another theoretical speculation might be that CCL11 could constrain recruitment and homing of endothelial progenitor cells worsening endothelial dysfunction and vascular regeneration in AH.26 Additionally, CCL11 might possibly increase vascular permeability, downregulate tight junction proteins, increase oxidative stress, and activate MAPK p38, Stat3, and NF-kB pathways in endothelial cells.27
Another significant finding of our study is the lower blood GDF11 levels in hypertensive patients compared with age and sex matched normotensives. Recent papers show GDF11 is not decreased in the circulation of aged rodents or older humans and that GDF11 is actually deleterious toward muscle repair in mice.28 In apolipoprotein E-null mice model was shown, that GDF11 protects against endothelial Injury and reduces atherosclerotic lesion formation.29 GDF11 activates both smad1/5/8 and smad2/3 signals in human umbilical vein endothelial cells30 and it supports migration and sprouting of endothelial progenitor cells.31 Further investigations are needed to clarify whether, and under what, if any, conditions GDF11 is a factor in the pathophysiology of human hypertension.
We are aware of the limitations of this exploratory study. Our study population was limited by its size, ethnicity and sex of the participants. We therefore cannot assume that they are representative of the wider population. Therefore, our findings should be confirmed in further trials in other independent hypertensive cohorts and explored in the general population.
None.
The authors have no financial or conflicts of interest to disclose.

©2018 Kuznik, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.