MOJ
eISSN: 2574-8130


Research Article Volume 4 Issue 1
Research Institute of Biology, VN Karazin Kharkiv National University, Ukraine
Correspondence:
Received: January 25, 2019 | Published: February 1, 2019
Citation: Ohiienko SL, Bondar AY, Ivanov EG, et al. Liver fibrosis has a different effect on the “lifespan” of lymphocytes and neutrophils in the in vitro system isolated from the bone marrow of young and old rats. MOJ Gerontol Ger. 2019;4(1):36-40. DOI: 10.15406/mojgg.2019.04.00174
Background: The content of lymphocytes and neutrophils in the bone marrow of young (3 months) and old (20 months) rats was investigated. The ability of lymphocytes to proliferate after transferring the bone marrow cells of young and old animals to the primary culture and the "lifespan" of neutrophils in culture, as well as the effect of liver fibrosis in young and old animals on the studied cell characteristics was determined.
Methods: Experiments were performed on male Wistar rats two age groups: young (3 month old) and old (20 month old) ones. Animals were divided into groups: an intact control group, a group with Cu–induced liver fibrosis and a group with CCl4–induced liver fibrosis. Cu–induced fibrosis was induced by repeated administration of copper sulphate, CCl4–induced fibrosis was induced by multiple intraperitoneally administration per chloromethane mixed with olive oil. Bone marrow cells were isolated from the 2 femoral bones of the rat, 8 animals were used in each variant. Cells cultured in medium 199 with HEPES and 20% inactivated fetal calf serum and antibiotics.
Results: The content of lymphocytes in the bone marrow of old animals was 167% more than in young ones. Induction of liver fibrosis with copper sulfate increased the lymphocyte count in the bone marrow in young animals by 167% and in old animals only by 26%. While the induction of fibrosis with carbon tetrachloride increased the content of lymphocytes in young animals by 71%, and in old animals, on the contrary, decreased their number by 33%. The "lifespan" of neutrophils isolated from old animals was higher in the primary culture than from neutrophils obtained from young animals. Liver fibrosis reduced the "lifespan" of neutrophils in culture. Possible mechanisms of this phenomenon are discussed.
Keywords: aging, bone marrow, liver fibrosis, lymphocytes, neutrophils
Long-term studies of the mechanisms of ontogenetic changes at various sites allowed us to formulate the central paradigm of gerontology. The essence of this is that in the process of ontogenesis, animals lose the ability to “adequately” (quickly and correctly) form adaptive responses to a complex of endogenous and exogenous factors.1-4 Some experts believe that this is due to the loss of reliability of functioning of body systems.5,6 This paradigm is so logical that few researchers questioned it and it seems that it does not require proof. However, this issue is of fundamental importance and knowledge of the mechanisms that underlie the loss of adaptability can be attributed to the central aspects of gerontology.
We believe that a successful experimental model for solving the issue of adaptive abilities of an organism in ontogenesis is the evaluation of the functional characteristics of the bone marrow in young and old animals. There is evidence that lipids accumulate in the bone marrow in a relatively large amount, and this leads to inhibition of its functions with age.7
The uniqueness of the bone marrow is that it very quickly and "subtly" responds to the constantly changing needs of the body in certain types of blood cells and the immune system. The performance of such functions is possible due to the structural organization of this fabric. In an adult, up to 4-5% of the body mass is the mass of the bone marrow; it is rich in hematopoietic stem cells and blood vessels.8 The blood vessels in the bone marrow occupy up to 50% of the whole mass of the bone marrow, and most of the vessels have a rather large diameter (to 500 microns), which allows cells formed in the bone marrow to penetrate into the circulatory system.
The proliferation rate and directions of differentiation of polypotent stem cells are determined by the characteristics of the microenvironment bone marrow cells, which may change during ontogenesis. The study of the cells microenvironment has shown that it is provided by: 1 - direct contact between hematopoietic stem cells and stromal cells (fibroblasts, adipocytes, epithelioid cells); 2-under the influence of various cytokines (colonies of stimulating factors - GM - CSF; G - CSF; M - CSF; interleukins - 3,1,6,7 etc.). 9,10 and other factors present in the blood, including cytotoxic ones, which can manifest themselves in various pathologies. It is shown that a change in the characteristics of the microenvironment leads to a violation of the speed and direction of differentiation and the development of certain pathologies, including oncology.11
It can be assumed that the change in the functional characteristics of bone marrow cells in older animals compared with young animals is because changes with age in other body systems affect the epigenetic and metabolic characteristics of bone marrow cells. It is known that highly differentiated bone marrow cells, which include lymphocytes, neutrophils, and others, have a short “lifespan” after entering the bloodstream.12
It can be assumed that the “lifespan” of the bone marrow cells will depend on the epigenetic and metabolic characteristics of the bone marrow, and they, in turn, will be determined by the microenvironment. If the microenvironment for bone marrow cells is different, then it can be expected that the "lifespan" of the cells will be different.
Previously, it has been shown that induction of liver fibrosis affects the behavior of bone marrow cells.13-15 If these assumptions are correct, then one can expect bone marrow cells obtained from young and old healthy (intact) animals and animals with a modified microenvironment, if they induce liver fibrosis by different inducers, then in their behavior, in particular, life in the in vitro system will be different. In other hand, if the "lifespan" of lymphocytes and neutrophils isolated from young and old animals is different, then this is determined by the characteristics of the age-dependent nature of the microenvironment of bone marrow cells. To test this, intact young (3 months) and intact old (20 months) animals, as well as animals after the induction of liver fibrosis, were injected with copper sulphate and carbon tetrachloride, bone marrow cells were isolated and the "lifespan" of lymphocytes, stab and segmented neutrophils in the primary culture of bone marrow cells.
Keeping of animals
A total of 48 mature male Wistar rats were separated into experiments. These rats were obtained from Research Institute of Biology, V.N. Karazin Kharkov National University (Kharkov, Ukraine). They were housed in a temperature controlled room (20–24°C) and adapted to a 12hlight/12 h dark cycle. The animals were given free access to food and water before and during the study. All experimental procedures employed were approved by and conducted in accordance with bioethical rules16 and with due consideration to circadian rhythms for the formation of biological responses. For 24 hours preceding isolation of bone marrow cells, animals did not receive any food. Removing animals from the experiment was always carried out from 8 to 10 a.m. local time.
The rats were divided into two age groups: 24 young (3 month old) and 24 old (20 month old) ones. In each age group, animals were divided into groups: an intact control group (n=8), a group with Cu–induced liver fibrosis (n=8) and a group with CCl4–induced liver fibrosis (n=8). Rat models of Cu–induced liver fibrosis were established by multiple intraperitoneally injection of copper sulphate in different doses (1–1, 25 mg/100 g of body weight), as in investigation.17 CCl4–induced liver fibrosis were induced by multiple intraperitoneally administration per chloromethane mixed with olive oil at a concentration of 0, 1 mL/100 g of body weight. The induction scheme is justified in the work.18 Rats injected with saline served as a control group.
Isolation and culture of bone marrow cells in vitro
Bone marrow cells were isolated from the 2 femoral bones of the rat by the method19 and cultured in medium 199 with HEPES and antibiotics (8% gentamicin and 8% streptomycin) and 20% inactivated fetal calf serum. Culture under standard conditions was carried out at 37 °C in the 5% atmosphere. The number of lymphocytes and neutrophils was determined after 48 and 96 hours of incubation of the bone marrow cells.20,21 Cytologic preparations were stained by Romanovsky–Giemsa, analyzed at 100–fold magnification with the Zeiss Primo Stari LED microscope (Germany).
Statistical analysis
Average deviation, standard deviation, standard error of the mean, sample size were used as characteristics of the samples. The statistical significance of the differences between the two groups of data was assessed using the nonparametric Mann–Whitney test. The statistical processing of the results was carried out using the Excel software. Differences between the control and trial data were considered reliable at p<0.05.
"Lifespan" of lymphocytes in the in vitro system during the cultivation of bone marrow obtained from young and old animals with liver fibrosis
The number of lymphocytes in the culture of young intact animals did not change during the culture process (Figure 1A). At the same time, the number of lymphocytes obtained from old animals, after transferring them to the in vitro system, to the 96th hour of culturing increased by 72% (Figure 1B). It should be noted that the number of lymphocytes in young intact animals was 8.25% of the total number of bone marrow cells, while at the same time, the proportion of lymphocytes in old animals accounted for more than 20% of the total number of bone marrow cells (Figure 1B).
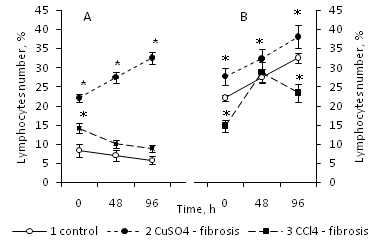
Figure 1 The number of lymphocytes in the primary culture, obtained from bone marrow of 3 month old intact animals (1), animals with Cu-induced (2) and CCl4-induced (3) liver fibrosis (A), and the same for the 20 month old animals of the same experimental variants (B).
Data percentage is expressed as mean, in relation to all identified cells, taked to 100%. Data were obtained of three independent experiments ±S.E.M, n=8 per group; p≤0.05 between liver fibrosis and control animals are determined by *, by Mann—Whitney’s U-test.
Consequently, lymphocytes obtained from intact old animals are able to fission in culture, unlike lymphocytes of young animals. Provided that lymphocytes were obtained from young animals with Cu–induced liver fibrosis, they actively proliferated in the in vitro system (their number increased by 47% (Figure 1A). However, if they were isolated from young animals with CCl4–induced liver fibrosis their amount decreased insignificantly for 48 hours and did not change any more till the end of the 96 hour period of culture as well as in the intact control (Figure 1A).
If lymphocytes were isolated from old animals with Cu–induced liver fibrosis, their number increased during the culturing process, as well as the lymphocytes of young animals (As shown in Figure 1B). Lymphocytes obtained from old animals with CCl4–induced liver fibrosis also proliferated, in contrast to those from the young animals, and their number increased by 96% to the 48th hour of culturing, after that their amount in culture decreased slightly (Figure 1B).
Consequently, the fibrosis affected the ability of lymphocytes to proliferate in culture and this depended on the age of the donor and inducer of fibrosis.
"Lifespan" of neutrophils in the in vitro system during the cultivation of bone marrow obtained from young and old animals with liver fibrosis
As is known, neutrophils have a relatively short lifespan.22 After 48 hours of in vitro culture the number of stab neutrophils obtained from intact young animals remained at the control level, and by the 96th hour of culture their amount decreased by 36% compared to the initial level (Figure 2A).
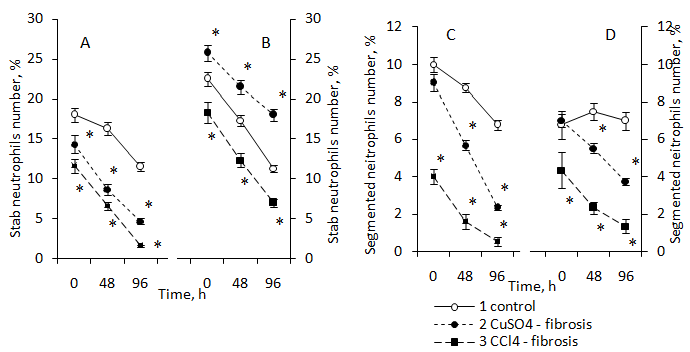
Figure 2 The number of stab neutrophils in the primary culture, obtained from bone marrow of 3 month old intact animals (1), animals with Cu-induced (2) and CCl4-induced (3) liver fibrosis (A), and the same for the 20 month old animals of the same experimental variants (B), as well as segmented neutrophils of 3 month old (C) and 20 month old (D) animals, respectively.
Data percentage is expressed as mean, in relation to all identified cells, taked to 100%. Data were obtained of three independent experiments±S.E.M, n=8 per group; p≤0.05 between liver fibrosis and control animals are determined by *, by Mann—Whitney’s U-test.
As is well known, stab neutrophils are maturing cell types. Their number in the bone marrow of young animals was 18%, and in the bone marrow of old animals–22,5 %. When they were transferred as part of the cell population to a culture isolated from intact young animals, their number did not significantly change. After 48 hours of incubation, their number decreased by 36 % to 96 hours of cultivation. At the same time, the lifespan of the stab neutrophils in culture obtained from old animals was shorter compared with those cells isolated from young animals (Figure 2A & Figure 2B).
Such differences in the "lifespan" of stab neutrophils in young and old animals may be related to the rate of their maturation and differentiation into segmented neutrophils. It turned out that the number of segmented neutrophils isolated from the bone marrow of young animals and transferred to culture decreased almost linearly from 0 to 96 hours of cultivation, i.e. these cells had a short lifespan. At the same time, segmented neutrophils isolated from old animals and transferred to culture had a much longer “lifespan” compared with those cells isolated from young animals (Figure 2C & Figure 2D).
The results may indicate a different rate of maturation of neutrophils in both young and old animals. It may also indicate age differences in functional characteristics.
The presence of liver fibrosis in young and old animals was accompanied by a decrease in the lifetime of neutrophils in culture, as evidenced by data on a sharp decrease in the number of cells in the primary culture (Figure 2). However, the "lifespan" of neutrophils isolated from young and old animals was different. If we compare the number of neutrophils of young and old animals that survived to the hour 96 of culturing in vitro, we can conclude that the "lifespan" of neutrophils in culture obtained from old animals was significantly longer than that of neutrophils obtained from young animals with the exception of stab neutrophils from intact animals (Figure 3).
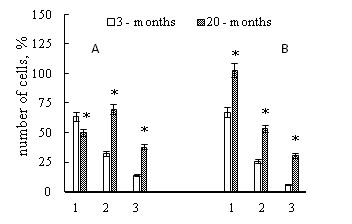
Figure 3 The number of stab neutrophils (A) and segmented nuclear (B) neutrophils that remained in the culture after 96 hours of culturing in vitro for intact animals (1), for animals with Cu – induced liver fibrosis (2) and CCl4 – induced liver fibrosis (3), which were obtained from young and old animals.
Data percentage is expressed as mean, in relation to all identified cells, taked to 100%. Data were obtained of three independent experiments, n=8 per group; p≤0.05 between liver fibrosis and control animals are determined by *, by Mann—Whitney’s U-test.
The presence of liver fibrosis reduced the "lifespan" of neutrophils, compared with intact animals in both young and old animals, with the exception of Cu – induced liver fibrosis in old animals (Figure 3).
The results of the work showed
The number of lymphocytes in the bone marrow of old animals (20 months of rats) was 167% more compared to the number of lymphocytes in the bone marrow of young animals. Induction of liver fibrosis with different agents in different degrees increased the number of lymphocytes in the bone marrow in young animals (3 months of rats) and old (20 months of rats) animals.
Administration of copper sulfate to young animals increased the content of lymphocytes in the bone marrow by 167% compared with the control level, and in older animals by 26%. The content of lymphocytes during the induction of fibrosis by carbon tetrachloride in young animals increased only by 71%, while in older animals it was, on the contrary, reduced by 33%.
It is known, that the number of B - lymphocytes in the bone marrow does not decrease with age.23 It should be noted that the number of autoantibodies24 is increased in old animals. An increase in the number of the total lymphocyte population in 20 months rats can reflect the action of factors (inflammatory process, infectious carriers, etc.) in old animals. In favor of the participation of lymphocytes in the adaptive response, there is evidence of an increase in lymphopoiesis in animals with liver fibrosis. However, in old animals that initially had a high level of lymphocytes in the bone marrow, induced by carbon tetrachloride did not increase their content. Consequently, the adaptive response of the organism to the introduction of the factor depends on the state of the functional system at the time of exposure, and it was different in young and old animals.
Lymphocytes obtained from old animals had a significantly greater proliferative activity in culture in vitro than lymphocytes isolated from the bone marrow of young animals. Lymphocytes are the only blood cell type that is capable of proliferating in peripheral tissues and this ensures their long-term existence and the formation of a long-term immune response.25 As is well known, the entry of lymphocytes into the cell cycle links extracellular mitogens to membrane receptors.26,27 which stimulate MAP - activation kinase cascade. As a result, several mechanisms can be triggered, in particular, the activation of the Myc genes and the expression of the genes encoding the family of cyclins.28 An important step in the passage of lymphocytes of the S - period of the cell cycle are the regulation factors E2F.29
It should be noted that the regulation of lymphocyte proliferation involves not only mitogens and the expression of the corresponding genes that ensure the functioning of regulatory cascades of the proliferative response, but also the state of the epigenetic characteristics of the genome. It has been shown that the dephosphorylating of genes can influence the proliferative activity of cells.30 The results suggest that with age there was a "change" in the microenvironment of bone marrow cells, which influenced the epigenetic and metabolic characteristics of bone marrow cells. Such differences did not affect (did not affect) the morphotypes of the cells, however, they influenced their functional features. In particular, on the "lifespan" of cells.
It was found that the "lifespan" of neutrophils isolated from old animals in the in vitro system was longer than that of lymphocytes in young animals. As already noted, neutrophils have a short lifespan. Cell longevity is determined by at least two global factors. Structural and functional organization of the cell and factors of its environment. Since neutrophils and leukocytes were isolated from young and old animals and placed in the same conditions in vitro, it can be assumed that the differences in their lifespan are due to different structural and functional features formed by the cells in the bone marrow, and above all, these are epigenetic and metabolic characteristics that are formed against this background. To explain the results obtained, the following working hypothesis can be expressed. The behavior of bone marrow cells, i.e. their ability to proliferate, "lifespan" and other functional characteristics depend on the epigenetic and, as a result, emerging metabolic characteristics of cells. The formation of the epinegotype is determined by the features of the microenvironment of cells, which is different for young and old, intact and animals with pathologies. Consequently, within a single morphological class, cells can vary greatly in their functional characteristics. The functional heterogeneity of bone marrow cells in older animals can be expressed to a much greater degree than in younger animals.
None.
No conflicts of interest has been declared by the author.

©2019 Ohiienko, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.