MOJ
eISSN: 2574-8130


Research Article Volume 1 Issue 2
1Department of Family Medicine, Abant Izzet Baysal University, Turkey
2Golyuzu Family Health Center, Turkey
Correspondence: Sebahat Gücük, Department of Family Medicine, Abant Izzet Baysal University, Bolu, Turkey, Tel 5056748192
Received: March 08, 2017 | Published: April 5, 2017
Citation: Gucuk S, Erkuran N. Improving the effects of pedometer use in individuals 65 years of age and older, under the supervision of family physician. MOJ Gerontol Ger. 2017;1(2):48-52. DOI: 10.15406/mojgg.2017.01.00011
Objective: Pedometer use in elderly patients, and assessing the possible potentiation of the effects of pedometer use by close follow-up of the patient. The aim of this study was to assess changes in physical activity among elderly individuals based on pedometer readings and motivational interviews
Methods: The study was conducted with individuals 65 years of age and older. Group 1 (pedometer+interview group) comprised 41 individuals, and group 2 (pedometer only group), 36 individuals. The participants’ anthropometric measurements, counts of GDS and steps were compared.
Results: Final GDS was significantly lower in group 1 than in group 2 (p<0.001). At the end of the 12-month study, the BMI values were lower than at baseline (28.19±2.89 vs. 28.73±14.94 kg/m2; p<0.001) in group 1 and higher in group 2 (27.77±2.72 vs. 27.69±2.77kg/m2; p=0.033). The WHR change was higher in group 1 than in group 2 (0.02±0.04 vs. 0±0.04 cm; z:-5.051, p<0.001).
Conclusion: This represents the first study to our country in primary care .Regular walking should be part of daily life in people of all ages, but especially in elderly individuals to prevent negative effects that can occur with increasing age. Primary care physicians can play an important role in encouraging regular free walking by their patients by emphasizing its protective and remedial effects
Keywords: family physician, older adults, pedometer, primary health care, regular control
BMI, body mass index; WHR, waist/hip ratio; GDS, geriatric depression scale
According to the World Health Organization, old age entails a gradual decrease in the ability to adjust to environmental factors, with individuals aged 65 years and older defined as old. The sociological, biological, and psychological decline is accompanied by changes in functional capacity induced by decreased cardiovascular, respiratory, and metabolic functioning.1,2 Physical activity is beneficial for health and well-being in old age. However, physical activity has been shown to decrease as adults become older.3 During old age, there may also be changes in social, economic, and health status as well as in family structure. The loss of a spouse or close friends or relatives can lead to loneliness, an inability to cope with daily life; and a decrease in social support, which together can result in depression.4 An alternative approach that has yielded highly positive results in the management of depression in elderly individuals is exercise.5 Regular exercise provides benefits to the elderly not only in terms of eliminating the depressive mood, but also in terms of several other aspects. Some of the guidelines prepared in compliance with the WHO’s suggestions recommend that individuals aged 65 and older should do medium density physical activities for a total of 300 minutes per week or similar physical activities in various durations and densities in order for the physical activity to be sufficiently beneficial for health.6 Nonetheless, many older individuals hesitate to exercise because they fear the risk of injury; however, this is not the case with walking.7
Simple and affordable pedometers are generally considered practical for individual- and population-based applications, as they provide a summary of the daily number of steps.8 Although pedometers are widely used in many countries to measure physical activity, their use in Turkey is very limited. However, the Republic of Turkey Ministry of Health, with the support of the country’s primary care healthcare professionals, has been conducting several projects aimed at encouraging exercise and greater physical activity in individuals of all ages.
The aim of this study was to assess changes in physical activity among elderly individuals based on pedometer readings and motivational interviews. The primary outcome measures were weight change, body mass index (BMI), and waist/hip ratio (WHR). As a secondary outcome measure, scores on the Geriatric Depression Scale (GDS) were determined.
Our randomized controlled study was conducted in patients 65 years of age and older who were registered at the Bolu Izzet Baysal Family Health Center (Turkey)) between April 2014 and March 2015. Of the 395 registered patients over 65 years of age were contacted by telephone and invited to participate in the study, resulting in 130 participants. Based on the order of their enrollment, they were randomized into two groups. A total of 77 of these participants, who were randomized in 2 groups according to the order of registration, were able to complete the study (Figure 1).
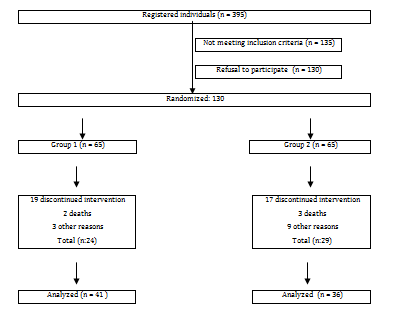
Figure 1 N=57; Epidemiological distribution of the pathological fractures, traumatic fractures, and nonunion.
The participants were first asked to complete a questionnaire about socio-demographic characteristics, a mini mental-status exam9 and a short physical performance battery10 to evaluate physical function. All participants were administered a geriatric depression scale of 30 questions (GDS-30).11 Patients receiving depression-related psychiatric treatment or who, in our opinion, should receive such treatment, patients scoring<23 points on the mini mental-status exam, those whose static balance (eyes open and closed) was suboptimal, and those not willing to participate were excluded from the study. Patient height and weight was measured. BMI was calculated as weight (kg)/height (m2)). WHR was measured in cm based on the waist and hip circumference, determined using a tape measure.12
Initially, each participant was given nutrition and exercise training, together with a 7-day retroactive TNV 3d® pedometer. The pedometers were supplied at no cost to the patients, in accordance with the Ministry of Health’s instructions. During the 12-month monitoring period, the patients were asked to keep the pedometers on a belt over one hip 7 days/week, except when showering or bathing. As in the Hernandez et al. study, both groups were checked up on every 2 months in the clinic or during house visits.13 From their pedometers, the recorded average data, the GDS scores, the weight BMI, and the WHY values of the latest week were calculated. In addition, bi-weekly telephone interviews were conducted with participants in the pedometer + interview group (Group 1), where patients were asked about their current exercise status. During the interview, the most recent number of steps was evaluated and discussed with the patient, without any time constraints, and advice was given regarding potential sources of confusion or improvement. For the patients in the pedometer only group (group 2), only their pedometer and other measurements were taken, while they were not provided with this additional motivation.
Our study was conducted using volunteers as participants, and permission was received from our local health authority.
The data were analyzed using SPSS version 20 for Windows (SPSS, Chicago IL, USA). For variables with normal distribution, the Shapiro–Wilks test was applied. For variables that were not normally distributed, differences between groups were determined using the Mann–Whitney U- and Kruskal–Wallis H-tests. Because the n values were >20, standardized z values were used for the Mann–Whitney U-test. Significant differences identified with the Kruskal–Wallis H-test were further analyzed using a post hoc multiple comparison test. The relationships between groups in categorical variables were assessed using a χ2 test. When the values expected in the cells of the 2 × 2 tables were too small, Fisher’s exact test was applied. For the R × C tables, a Pearson χ2 analysis was applied using a Monte Carlo simulation. A p-value<0.05 was considered to indicate statistical significance.
Some characteristics of both groups in the initial stages of the study were shown in (Table 1). The average age of the group 1 patients was 72.44±5.41 years, and that of the group 2 patients 74.28±4.79 years. None of the participants was actively employed. In groups 1 and 2, 65.85% (n=27), and 71.43% (n=28), respectively, had been previously employed but were retired at the time of the study. There was no difference between groups with respect to sex, education level, marital status, domestic status, residence, and physical exercise frequency (p>0.05).
Characteristics |
Group 1 (n = 41) |
Group 2 (n = 36) |
P-value |
||
Sex |
Female |
22 (53.66%) |
19 (52.78%) |
0.938 |
|
Male |
19 (46.34%) |
17 (47.22%) |
|||
Marital status |
Married |
25 (61%) |
19 (52.8%) |
0.712 |
|
Widowed |
14 (34.1%) |
14 (38.9%) |
|||
Divorced |
2 (4.9%) |
3 (8.3%) |
|||
Domestic status |
Alone |
4 (9.8%) |
3 (8.3%) |
0.121 |
|
With spouse |
16 (39%) |
13 (36.1%) |
|||
With spouse and children |
16 (39%) |
7 (19.4%) |
|||
|
4 (9.8%) |
11 (30.6%) |
|||
Other |
1 (2.4%) |
2 (5.6%) |
|||
Any diagnosed chronic |
Yes |
38 (92.7%) |
29 (80.6%) |
0.175 |
|
No |
3 (7.3%) |
7 (19.4%) |
|||
Residence |
House |
19 (46.34%) |
19 (52.78 |
0.573 |
|
Condo |
22 (53.66%) |
17 (47.22%) |
|||
Frequency of physical exercise |
None |
24(%58,5) |
13(36,1) |
0.134 |
|
1–2 times a week |
14(34,1) |
17(47,2) |
|||
3 or more times a week |
3(%7,3) |
6(16,7) |
|||
Table 1 Baseline characteristics of the participants.
|
Group 1 n = 41 (mean ± SD%) |
Group 2 n = 36 (mean ± SD%) |
z |
p |
|
GDS total |
Beginning |
8.9 ± 3.12 |
9.83 ± 2.44 |
−1.552 |
0.121 |
12 months |
7.73 ± 2.17 |
10.72 ± 2.99 |
−4.25 |
0.001 |
|
Weight (kg) |
Beginning |
76.23 ± 8.9 |
74.67 ± 7.41 |
−0.297 |
0.767 |
12 months |
75.09 ± 8.22 |
72.47 ± 12.36 |
−0.179 |
0.858 |
|
BMI (kg/m2) |
Beginning |
28.73 ± 3.2 |
27.69 ± 2.77 |
−1.389 |
0.165 |
12 months |
28.19 ± 2.89 |
27.77 ± 2.72 |
−0.633 |
0.527 |
|
Waist/hip ratio |
Beginning |
0.99 ± 0.17 |
0.92 ± 0.1 |
−1.737 |
0.082 |
12 months months |
0.97 ± 0.16 |
0.93 ± 0.08 |
−0.684 |
0.494 |
|
Baseline pedometer readings over 7 days: mean number of stepsa |
Beginning |
4464.93 ± 725.43 |
4390.28 ± 684.26 |
−0.546 |
0.585 |
12 months |
5476.08 ± 1359.14 |
5380.65 ± 1317.65 |
−0.3 |
0.764 |
|
Table 2 Post-study changes among groups.
*p < 0.05.
aMean number of steps = total number of steps recorded by the pedometer divided by the number of days.
GDS, geriatric depression score; BM
The initial average GDS score was not significantly between two groups (p=0.121). But the final score was significantly lower in group 1 than in group 2 (p<0.001). The GDS in group 2 was higher at the end of the study than at baseline p<0.001). In parallel with the increase in the average number of steps recorded at the bimonthly measurements, the average GDS decreased. In the post hoc analysis, although this relation was not a strong one at the final interview, the correlation was confirmed as negative (r:−0.248, p=0.038).
Average weight loss in group 1 (1.14±1.35kg) was less (z:−4.601; p=0.001) than that in group 2 (2.2±10.9kg), but at the end of 12 months, when current weights within groups were compared with their initial weights, there was a meaningful weight loss in the 1st Group. p< 0.001) At the end of the 12-month study, the BMI values were lower than at baseline (28.19±2.89 vs. 28.73±14.94kg/m2; p<0.001) in group 1 and higher in group 2 (27.77±2.72 vs. 27.69 2.77kg/m2; p=0.033). The WHR change was higher in group 1 than in group 2 (0.02±0.04 vs. 0±0.04 cm; z:-5.051, p<0.001). Furthermore, WHR in group 1 patients decreased between the beginning and end of the study (p<0.001), whereas that in group 2 increased (p=0.009).
The average number of steps taken was higher at all time points in group 1 than in group 2, as was the average number steps at the end of 12 months (5476±1359.14 steps/day) compared to the baseline value (4464 725.43 steps/day; p<0.001). The change in the number of steps/day over the 12-month period was 1011.15±834.81 in group 1 and 990.37±825.11 in group 2 (z:−0.015; p=0.988) (Table 2) Even though the difference between the final and initial values of the groups were meaningful, when the two groups were compared with each other, no difference was observed with regards to the average number of steps. (p:0.764).
Regular walking plays an important role in increasing the activity level of sedentary people and therefore in improving the health of society. It is a simple form of exercise that can be performed by individuals of all ages and socio-economic groups, and by women as well as men. Moreover, walking is a life-long form of exercise that can maintain general health over the long term. In fact, it is the most frequently prescribed non-pharmacologic treatment in the world.14
To measure physical activity independent of patients’ subjective self-report, pedometers have been introduced into clinical studies. Their advantages include their small size, relatively low cost,15,16 and the positive feedback they provide with respect to the daily numbers of steps taken.17 Therefore, we decided to measure the level of physical exercise of our elderly patients via pedometers. Furthermore, we tried to find out whether their physical activities could be improved by conducting close follow-ups and motivating them through interviews.
Randomized studies and meta-analyses have confirmed the anti-depressant affect of physical activity and exercise in older adults with clinical depression.18 Physical activity was also shown to have long-term effects in improving mood19 and in reducing depressive symptoms.20 Moderate aerobic activity, such as walking,21 has positive effects, as does progressive resistance training. Furthermore, these positive effects result in an increase in the frequency of the respective activities and increase with longer duration of the intervention.22In our study, the GDS values of the participants in the 1st Group were significantly better than the other group. Furthermore, a correlation was found between the increased number of steps and the decreased GDS scores according to the assessment that was conducted every 2 months with this group. However, even though the number of steps showed an increase in the 2nd group, there was no decrease in their GDS scores. Therefore, it is plausible to think that a decrease in the GDS scores in the 1st group can be explained not only with the increase in number of steps, but also with the possible effects of phone calls conducted every 2 weeks with this group, along with the suggestions made, and the attention shown.
Excessive visceral fat and central obesity are related to several cardiovascular diseases and to mortality.23 The WHR, a measure of relative fat distribution, has been used to determine health risks.24 Duchečková reported high WHR values in individuals with a low level of physical activity.25 In a population-based study, the number of steps taken was shown to be inversely related to low BMI and low WHR. Similarly, another study found that the number of steps/day was inversely related to abdominal obesity.26 Our results are in agreement with those of other studies. Considering our study from these points of view, there was no difference between the two groups regarding loss of weight and the changes in the BMI values, while the groups were significantly better than their initial values. In terms of WHR, while there was a meaningful decrease in the 1st Group compared to the 2nd, an increase in the WHR was observed in the 2nd Group. Considering the fact that all these values are significant in terms of fat distribution and the general cardiovascular health, it can be said that physical activity with a pedometer is beneficial. However, it is also true that the interviews conducted with the 1st Group had a minimal effect on these values.
It was stated that regardless of the well-known benefits of physical activities, especially the 45% of the population over 65 of age do not meet the recommended levels of physical activity. This is a rapidly increasing problem as the number of people aged over 65years in the world is expected to triple in the next 30 years. 300 minutes of medium density aerobic physical activity per week is recommended for the elderly in order to bring out adequate benefits of exercising.6 In their study of older adults, Rowe et al. recommended an average of 7000–8000 steps/day.27 In Turkey, where primary care health services are steadily improving, the Ministry of Health has developed several motivational programs aimed at elderly people but appropriate for every age group. In our study, the participants’ average number of steps were between 5000 and 6000. Following the introduction of a pedometer, by the end of the study, the number of steps increased in participants; the increase from the initial values was significant in group 1 as well as group 2. Thus, our results show that the support provided simply by giving patients a pedometer and contacting them via regular interviews leads to an increase in the number of steps taken. The absence of a significant difference in the number of steps taken by groups 1 and 2 independent of the training provided might have reflected a limit for increasing the number of steps in this relatively inactive age group. However, this also demonstrates the importance and motivational effect of encouragement by health professionals.
The results of the present study may also be applicable to the Turkish population. However, a limitation of our study was the small number of patients representing a single geographic area. Also, our patients had no serious mobility problems, and had good cognitive function and adequate verbal communication. Furthermore, their physical activities were monitored only in terms of the number of steps, and their walking speed was not standardized. Our study will encourage other studies with a longer duration of monitoring and wider participation, in addition to raising awareness of the positive effects of walking in elderly individuals.
Regular walking should be part of one’s life style during every period of life, but it is especially important in preventing the negative effects that typically occur with increasing age. In Turkey, health policies are currently being developed to encourage physical exercise. As primary care physicians, we should recommend regular free walking to our patients, given its protective and remedial effects, and encourage all members of the population to walk regularly.
This study did not receive any specific funding or grants. The authors have no conflicts of interest to declare.
None.
Author declares there is no conflict of interest in publishing the article.

©2017 Gucuk, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.