MOJ
eISSN: 2574-8130


Review Article Volume 7 Issue 3
Nutrition Department, Faculty of Floriano – FAESF, Brazil
Correspondence: Nicole Debia, Professor, Nutrition Department, Faculty of Floriano – FAESF, Floriano-PI, Brazil
Received: May 01, 2022 | Published: September 30, 2022
Citation: Ageme AS, da Silva WB, Debia N. Diagnosis of sarcopenia and nutritional intervention in the elderly: literature review. MOJ Gerontol Ger. 2022;7(3):65-70. DOI: 10.15406/mojgg.2022.07.00294
Sarcopenia is a skeletal muscle disorder that affects the elderly, characterized by loss of muscle volume associated with loss of strength and performance, increasing the risk of fractures, falls, physical disability and mortality. The diagnosis includes DEXA, physical performance tests, anthropometric measurements as calf circumference and strength test using handgrip strenght that check muscle volume, it’s quality and the elderly mobility and balance hability. Nutritional intervention is an essential strategy in the control of sarcopenia and comprises the adequate energy and protein intake, in addition to vitamin D, primarily. In this context, this narrative review aimed to collect current information about the characteristics of sarcopenia, diagnosis methods and nutritional strategies for the treatment. Updates were searched in BVS, PubMed and SciELO databases, technical books, and Brazilian and international consensus. The structured content makes consultation and updating on sarcopenia easier, from diagnosis to the most relevant dietary recommendations for the treatment of the disease.
Keywords: diagnosis, elderly nutrition, dietary recommendations, sarcopenia, treatment
Sarcopenia is defined as a progressive and generalized skeletal muscle disorder associated with an increased probability of fractures, falls, physical disability and mortality, a definition used worldwide that allows advances in the identification and care of affected patients.1 It can be considered a public health problem due to the social implications, such as dependence, and its impact on health policies, in addition to the increase in expenses for the health system and for the State.2
It is characterized by being a more frequent condition in the elderly, but it also affects a considerable part of hospitalized patients. Furthermore, sarcopenia can also be a comorbidity resulting from some diseases such as chronic obstructive pulmonary disease (COPD) resulting from persistent airway obstruction with the presence of emphysema and/or chronic bronchitis. The inflammatory manifestation of the disease directly affects the musculoskeletal system, causing muscle atrophy, osteoporosis, muscle depletion and decline in functional capacity.3
Other risk factors may also predispose the elderly to sarcopenia, such as the presence of fractures and the need for immobilization. Furthermore, changes in body composition resulting from the physiology of aging itself, such as a reduction in body water content, an increase in fat content and a decline in skeletal muscle mass can lead to sarcopenic conditions.
A large study called FIBRA (Fragility in Elderly Brazilians) studied frailty conditions in urban residents of seven cities in six Brazilian states and related them to demographic, socioeconomic, physical and psychological aspects, performance in daily activities, care expectancy, depressive symptoms and life satisfaction. In this study, frequency data were collected for the indicators of frailty, weight loss, fatigue, low hand grip strength, slow gait, physical inactivity and cognitive level. Among the most relevant results are unintentional weight loss in 25.5% of the population of Parnaíba/PI, in addition to reports of fatigue in 28.7% of Parnaíba/PI and Campina Grande/PB. The cities mentioned were the ones with the highest prevalence of cognitive impairment and the lowest GDP (gross domestic product) per capita. The opposite was observed in Poços de Caldas/MG and Ivoti/RS, cities that presented higher income and better preserved cognition.4
Association of sarcopenia with nutritional status
Malnutrition
Malnutrition, per se, is characterized as a risk factor for the development of sarcopenia, as it comprises the reduction of global food intake - anorexia of aging - and, consequently, the reduction of energy and protein intake, mainly. Associated with lack of exercise, it promotes the continuous loss of muscle mass, which leads to a decrease in strength and endurance.5,6 The main reasons that predispose the elderly to malnutrition are psychosocial factors such as widowhood, depression, social isolation, poverty that results in the purchase of less expensive and less nutritious food, in addition to food monotony.7
Impaired cognitive ability can result in inappetence or food refusal. Poor dentition quality is another contributing factor, as it compromises masticatory ability. Among the problems related to teething are the frequent presence of caries, periodontal diseases, poorly maintained dentures and edentulism. In this way, there will be a decrease in the consumption of fresh meat, fruits and vegetables due to the difficulty in chewing and inadequate consumption of energy, protein, iron, fiber and vitamins.8
Polypharmacy is also an indirect factor for malnutrition, as it can lead to hyposalivation. In these cases, the elderly will avoid the consumption of dry or hard foods and will prefer creamy and liquid preparations that naturally have lower caloric density.9 Other physiological changes in the oral mucosa, such as the prominence of the sebaceous glands, smooth appearance of the mucosal surface and reduction of the buccal and lingual epithelium, directly interfere with food consumption by increasing local sensitivity, causing burning sensation in the ingestion of acidic, spicy foods or with extreme temperatures.10 In these situations, nutritional supplementation should be considered.11
Iron deficiency anemia, in turn, may be a consequence of low intake of protein foods of animal origin, which contain high levels of heme iron. This condition is usually associated with sarcopenia and frailty syndrome, as reduced oxygen uptake by low hemoglobin levels lead to tiredness and decreased mobility.12
Obesity
Sarcopenic obesity in aging is defined as the loss of muscle mass in obese elderly people combined with low-grade systemic inflammation promoted by excess adipose tissue, which may be associated with insulin resistance, sedentary lifestyle and decreased concentration of growth hormones and testosterone. Nutritional intervention for obese elderly people in this condition seeks to reduce muscle and bone losses, preserve strength and flexibility through behavioral changes combined with physical exercises and food supplementation.13 Therefore, intentional weight loss is only recommended for older adults with comorbidities resulting from obesity and who may benefit from reduced body fat at the expense of a greater risk of loss of skeletal muscle mass.14
Granic et al. (2020) show us that current evidence points to factors that are related to lifestyle and that can be changed, such as physical exercise, proper diet and non-drug treatments to support muscle health. Thus, the present study aimed to describe the characteristics of sarcopenia, its relationship with nutrition, as well as the methods of detection and nutritional intervention in the elderly population.
Narrative literature review that broadly describes a subject from a contextual or theoretical point of view based on analysis and interpretation of scientific production, but without the intention of exhausting the topic. This type of research favors the identification of gaps for the development of new primary studies.15
To answer the central question “What nutritional strategies can be used for the prevention and treatment of sarcopenia in the elderly?” publications were searched in journals indexed in the following databases: Virtual Health Library (VHL), PubMed, SciELO (Scientific Electronic Library Online), in addition to textbooks with content relevant to the topic, consensus of Brazilian and international societies and associations.
The search was carried out between August 2021 and April 2022 using the descriptors 'Sarcopenia' (Sarcopenia), 'Elderly' (Elderly), 'Risk Factors', 'Prevention' and 'Dietary Intervention' ' (Dietary Intervention), with no date or study design. Publications not available in full were not considered.
Stages of sarcopenia
According to the latest consensus on sarcopenia in the elderly EWGSOP2 (European Working Group on Sarcopenia in Older People), the phenomenon canbe classified into three stages:16
Diagnostic methods
Research developed by McGregor et al.17 and Buckinx et al.18 showed a strong association between sarcopenia and poor muscle quality and quantity, with reduced strength as the main determinant for its investigation and diagnosis. Methods to aid in the diagnosis and analysis of muscle content include imaging tests, anthropometry and physical performance tests described below, with the respective cut-off points summarized in Table 1.
|
Parameter |
Cut points |
|
|
|
Men |
Women |
|
ALM (Kg) |
< 20 |
< 15 |
|
ALM/height2 (kg/m2) |
< 7.0 |
< 6.0 |
|
SARC-F (points) |
≥ 4 |
≥ 4 |
|
CP (cm) |
< 31 |
< 31 |
|
MLG (kg/m²) |
||
|
55 to 74 years old (P10) |
< 17.6 |
< 14.6 |
|
> 75 years (P10) |
< 16.9 |
< 13.7 |
|
FPP (kg/F) |
< 30 |
< 20 |
|
TUG (s) |
> 20 |
> 20 |
|
Test walk 400m (min) |
≥ 6 |
≥ 6 |
|
SPPB (points) |
< 7 |
< 7 |
Table 1 Cut-off points for the diagnosis of sarcopenia
Subtitle: ALM, appendicular skeletal muscle mass; SARC-F, simple questionnaire to rapidly diagnose Sarcopenia; CP, calf circumference; FFM, fat free mass; P10, 10th percentile; HGS, hand grip strength; TUG, time up and go; SPPB, short physical performance battery
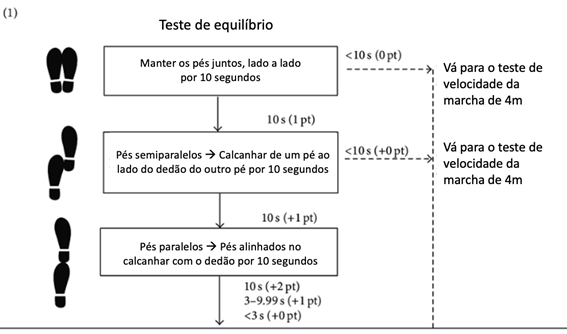
Figure 1 Short physical performance battery (SPPB) steps. Source: adapted from Guralnik et al.27
Nutritional intervention in sarcopenia
Energy nutrients
Carbohydrates are the main energy providers for tissues, especially brain cells, and their adequate intake maintains blood glucose during exercise, repairs muscle glycogen during recovery, and preserves muscle protein.28 It is suggested by the AMDR (Acceptable Macronutrient Distribution Range) that carbohydrate intake varies between 45% and 65% of the total caloric intake (TCV), respecting the minimum supply of 130g (IOM, 2002).
Lipids are more energetically dense and, although they are also essential, their intake should be monitored due to the risk of developing dyslipidemia, cardiovascular disease and excessive weight gain. The Institute of Medicine recommends that fat intake corresponds to 20% to 35% of the VCT, with priority given to long-chain polyunsaturated fatty acids of the omega 3 series.29
The estimated daily energy requirement must be calculated individually considering the variables 'age', 'weight', 'height' and level of daily physical activity30 (Tables 2 and 3).
Sex |
Equation (kcal/day) |
Male |
662 - (9.53 x I) + CAF x [(15.91 x P) + (539.6 x E)] |
Female |
354 - (6.91 x I) + CAF x [(9.36 x P) + (726 x E)] |
Table 2 Predictive equations of daily energy requirement
Source:IOM, 2002.
Caption: kcal, kilocalories; I, age; CAF, physical activity coefficient; P, weight; AND, stature
Sex |
Inactive or sedentary |
Little active |
Active |
very active |
Male |
1 |
1.11 |
1.25 |
1.48 |
Female |
1 |
1.12 |
1.27 |
1.45 |
Table 3 Physical activity coefficients
Source: IOM, 2002.
Total proteins
Adequate protein intake positively impacts the increase and regeneration of muscle protein content, since the maintenance of skeletal lean mass is the result of the balance between catabolism and protein synthesis.31 In the elderly, one of the ways to reach the dietary protein quota is through whey protein supplementation, as it is rapidly absorbed, contains essential amino acids and a relevant leucine content, thus ensuring the stimulation of protein synthesis. muscle.32 Adequate protein consumption for the elderly is 1.0 to 1.5 g/kg/day, a recommendation proposed by the Society for Sarcopenia, Cachexia and Wasting Disease.33
Although the control of daily protein intake is essential, studies show that it must be well distributed throughout the day to ensure an optimized anabolic response. Paddon-Jones and Rasmussen34 found that the intake of 30g of protein in the three main meals (breakfast, lunch and dinner) had a greater contribution to maximum protein synthesis when compared to the intake of 10g or 20g per meal. On the other hand, the consumption of 60g of protein at dinner showed no significant difference when compared to the consumption of 30g.
According to recommendations from the Food and Nutrition Board,30 the sum of the daily intake of essential amino acids should be equal to 262 mg for each gram of protein ingested.
Leucine
Leucine is an essential branched-chain amino acid (Figure 3) and one of its functions is to increase the phosphorylation of proteins that are involved in the regulation of muscle tissue formation.35 It can be used to enrich amino acid supplementation with the aim of delaying muscle loss, especially in the elderly who practice physical exercises.33 To promote an increase in protein synthesis after meals, an intake of more than 3g per day is suggested.13,36
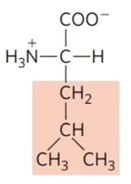
Figure 3 Chemical structure of leucine. The shaded structure comprises the radical that differs it from other amino acids.
Creatine
Creatine (α-methyl-guanidinoacetic acid) is an amine that belongs to the family of guanidino phosphagens, found exclusively in eukaryotic cells37 and which can be obtained from the diet or synthesized endogenously.38 Muscle mass gains by resistance exercises can be optimized with the use of it by the elderly. Consumption before or after exercise is more relevant than the amount ingested. It is suggested from 0.03g to 5.0g/kg per day39 or a total of 20g per day with the aim of attenuating muscle loss.40 The use of creatine monohydrate as a supplementation increases the availability of phosphocreatine, which is the form of energy storage in the muscle fiber for use in high intensity exercises.33
β-hydroxy-β-methylbutyrate (β-HMB)
β-HMB (or HMB) is a metabolite of leucine, which is widely used by athletes and bodybuilders to increase strength, muscle mass and physical performance in exercise.41 Although synthesized in the human body, only 5% leucine is converted into HMB, which would justify the isolated consumption of the metabolite.42 Oral HMB supplementation increases its concentration in plasma and intramuscular vessels and is effective in preventing the reduction of muscle mass at rest.43 A study on the effects of HMB supplementation on lean mass in bedridden elderly showed that the intake of 3g/day for five days before being bedridden and ten days during rest attenuated muscle loss.44 In a review developed by Cruz-Jentoft et al.16 it was found that supplementation of essential amino acids together with HMB had anabolic properties and positive effects on parameters of muscle mass and function. Although the findings are relevant in individuals with short-term muscle disuse, in the example of a hospitalization.
The mechanisms of action proposed for the effect of HMB on muscle mass include increased membrane integrity of muscle cells, increased protein synthesis via m-TOR (mammalian target of rapamycin, a cellular protein with a central role in cell growth, proliferation and maintenance) and reduced degradation of cellular proteins by inhibiting the ubiquitin-proteasome complex. However, there is still no consensus on the effectiveness of this supplementation.45
D vitamin
Deficiency of micronutrients such as selenium, magnesium and vitamins D and E are the most related to sarcopenia and weakness, as they have specific mechanisms that can affect muscle mass, with special attention to vitamin D.46
Vitamin 25(OH)D, or even hydroxyvitamin D, can be used as a supplement with the intention of attenuating sarcopenia in elderly people with proven deficiency. 25(OH)D levels below 30ng/mL already indicateneed for oral supplementation and doses of 50,000 IU of vitamin D3 per week are shown to be safe to correct deficiencies.33,40,47
Mechanisms of action of vitamin D in reducing the effects of sarcopenia includein improving the balance of calcium and potassium and consequent optimization of muscle contraction and cell regeneration.48–52 The recommendations of the main nutrients can be seen in Table 4.
Nutrient |
Recommendation |
Reference |
Energy (kcal) |
individual prediction |
IOM |
Carbohydrates (%VCT) |
45 to 65 |
IOM |
Total fat (%VCT) |
20 to 35 |
IOM |
Omega 3 fatty acids - EPA+DHA (mg) |
500 |
ISSFAL |
Total proteins (g/kg) |
1.0 to 1.5 |
IOM |
Essential amino acids (mg/g protein) |
||
Leucine (g) |
3 |
Johnson |
creatine (g) |
20 |
Wall |
HMB (g) |
3 |
Deutz |
Vitamin D (mcg) |
||
51 to 70 years |
15 |
IOM |
> 70 years |
20 |
IOM |
Table 4 Recommended daily nutrient intake
Caption: kcal, kilocalories; VCT, total daily caloric value; EPA, eicosapentaenoic fatty acid; DHA, docosahexaenoic fatty acid; HMB, β-hydroxy-β-methylbutyrate
This narrative review brought together methods of identification and nutritional intervention in sarcopenia, facilitating the consultation of updated information on the topic in the context of nutrition, mainly. The tables allow quick access to the cut-off points for the identification of the disorder, in addition to the recommendation of energy intake, macronutrients and some of their by-products, in addition to vitamin D, in order to optimize the nutritional behavior for the target audience.53–56
None.
The authors declare they have no conflicts of interest that are directly or indirectly related to the research.
None.

©2022 Ageme, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.