MOJ
eISSN: 2574-8130


Protocol Article Volume 3 Issue 6
1Department of Health Sciences of Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil
22Department of Physiotherapy, University of Franca, Franca, Brazil
Correspondence: Almir Resende Coelho, Post graduate Department of Biomechanics, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brazil
Received: October 24, 2018 | Published: November 14, 2018
Citation: Coelho AR, Abreu DCC. Effects of anchor system during balance rehabilitation in subjects with chronic dizziness of peripheral vestibular origin: a controlled, randomized, single-blind clinical trial. MOJ Gerontol Ger. 2018;3(6):414-420. DOI: 10.15406/mojgg.2018.03.00157
Background: With the increase of life expectancy and the subsequent development of otoneurologic symptoms, such as dizziness and vertigo, several intervention protocols that comprise the compensation, habituation, replacement and adaptation of the vestibular system have been elaborated. However, new protocols that use other components of postural control in patients with chronic dizziness of vestibular origin are necessary. Therefore, this study aims at evaluating the efficacy of anchor system during balance rehabilitation of patients with chronic dizziness of peripheral vestibular origin who have not responded positively to traditional vestibular rehabilitation, regarding quiet standing and dynamic postural control, gait and dizziness.
Methods/design: A randomized, single-blind, controlled trial with a three-month follow-up. The sample will consist of volunteers age 50 and over, both sexes, diagnosed with chronic dizziness of peripheral vestibular origin. Cognitive and functional assessments will be performed in all volunteers at the time of pre intervention, post intervention, and three-month follow-up. The primary outcomes will be the variables related to quiet standing and dynamic posturographic analysis based on displacement of center of pressure (COP) and limit of stability (LOS) measured by the Balance Master®, as well as functional assessments (Timed Up and Go test [TUG], sitting-rising test [SRT], Dynamic Gait Index [DGI] and Mini-BESTest). The secondary outcomes will be the data related to cognitive analysis (Mini-mental state examination, Clock Drawing Test, verbal fluency test and Trail Making Test B), dizziness evaluations and their aspects (Dizziness Handicap Inventory and Vestibular Disorders Activities of Daily Living Scale). The intervention will consist of two different protocols for the Balance Rehabilitation (BR), where the volunteers will be randomized into three groups: G1 - Proprioceptive exercises with anchors (n=20); G2 - Proprioceptive exercises with no anchors (n=20); and G3 - Control (n=20) without performing any exercise. The BR protocols will be supervised in groups, twice a week, with duration of 45 minutes and twelve sessions.
Discussion: Although the benefits of the Cawthorne-Cooksey vestibular rehabilitation protocol are well established in the literature, there is a need for new protocols which stimulate somatosensory, visual and cognitive components aimed at improving both balance and otoneurologic symptoms in patients with chronic seizures.
Keywords: aged, dizziness, postural balance, rehabilitation, vestibular diseases
The majority of symptoms reported by elderly people, such as lack of stability, loss of postural balance and dizziness, can be the consequence of several diseases derived from the vestibular system.1 Dizziness are prevalent in 5% to 10% of the world population, and they are the seventh most frequent complaint in women and the fourth in men. Approximately 47% of men and 61% of women over 70 years old are affected by dizziness. They are present in 65% of subjects aged 65 years and over, who live in the community, and in 81% to 91% of elderly people attended at geriatric outpatient clinics with 65 years and over.2
Because of aging, the vestibular system is affected by electrophysiological and structural changes which can generate consequences to postural control: synaptic changes in the vestibular nerve from the age of 40; signs of degeneration in the vestibular receptors of the semi-circular channels and saccule after the age of 50; and, after the age of 60, increase in friction of the nerve fibers of the vestibular nerve, leading to selective loss of density of myelinated fibers which determine the reduction of conduction velocity of electrical stimulation in the vestibular nerve; reduction of nystagmus response to caloric and rotation tests; and decrease in amplitude of optokinetic nystagmus and visual pursuit movement for stimuli speed, which can influence the performance of vestibular reflexes.3
Therefore, vestibular system disorders are important problem in the healthcare, because an increase in age is directly proportional to the presence of multiple otoneurologic systems, such as vertigo, dizziness, lack of body stability, gait disturbances and falls.4 Among the main therapies recommended for the treatment of vestibular disorders, as well as multiple otoneurologic symptoms, we can highlight drug therapies, surgery and vestibular rehabilitation (VR), a type of therapy characterized by its physiological action on the vestibular system, acting on the central mechanisms of neuroplasticity, generating adaptation, habituation and replacement mechanisms in the vestibular system aimed at achieving vestibular compensation.5
This compensation is obtained using the classic protocols elaborated by Cawthorne (1945), Cooksey (1946) and Norré and Deweerdt (1980), involving habituation and replacement exercises which allow the performance of vestibular system abilities related to both head movement velocity and correction of eye positioning to stabilize the visual field and reestablish head orientation. This result is possible by obtaining the visual paradigm which moves over the retina during head movements.
Thus, the therapeutic approaches aimed at keeping sensory, motor and perceptual abilities is crucial to carrying out everyday activities, as well as physical and leisure activities. The deterioration of these factors can be the reason for accidents caused by falls and their consequences, such as physical changes (tissue injuries, gait changes), psychological changes (fear of falling, depression), social changes (isolation, dependency), and economic changes (medication costs, rehabilitation). Therefore, it is important to search for effective ways to prevent falls, as well as clinically viable assessments which can be introduced in daily clinical practice for this population.
Mauerberg-Decastro 6 has developed an instrument aimed at improving the corporal stability called the anchor system. This system is based on the development of a non-rigid tool, which consist of flexible ropes attached to a weight on the extremity that touches the ground and another weight held in the user’s hands. The participant assumes the position of an anchor with this tool during activities with more postural control demand, without remove the weight from the ground.7
Similarly, the anchor system works as a mediator of haptic information between the ground and the participant's body. The haptic system, or touch feedback, works through an active exploration of the environment (static or dynamic), which involves the interpretation of spatiotemporal stimuli when they interact with several types of mechanoreceptors.8
The low function of the vestibular component of the sensory system, the central nervous system (CNS) delegates more ''responsibility'' to the other system components (vision and the somatosensory system), where the spatiotemporal and visual exploration are more valued by the CNS in keeping postural control.9
However, in the case of patients with vestibular disorders, there may be disorders of information processing, which generating conflict among the visual, vestibular and somatosensory systems, which could explain the permanency of symptoms of chronic vestibular disorder where patients may not be able to modulate the sensory information in a way that is adequate to ensure postural balance.10
Postural control, a complex ability which involves postural orientation and the maintenance of the subject’s balance, depends on central processing inputs related to visual, vestibular and somatosensory afferent mechanisms9 and the neuromuscular proportional action of efferent mechanisms. With the objective of keeping postural stability, information related to visual, vestibular and somatosensory systems should be integrated and selected in accordance with the environment and the type of task to be performed.1
The anchor system can help the vestibular system to achieve a new adjustment of the sensory mechanisms and/or improve the somatosensory system with the aim of reducing the disorders of information processing and then contributing to balance improvement.
This study aims at evaluating the efficacy of anchor system during balance rehabilitation of patients with chronic dizziness of peripheral vestibular origin who have not responded positively to traditional vestibular rehabilitation, regarding to quiet standing and dynamic postural control, gait and dizziness. The second objective of this work will be establish a proprioceptive exercise protocol to improve postural control in patients with chronic dizziness of peripheral vestibular origin, as well as to evaluate the maintenance of the benefits derived from the anchor system in these patients.
A controlled, randomized, single-blind, prospective cohort clinical trial with a three-month follow-up. This study was approved by the Ethics Committee on Human Research of the Ribeirão Preto Clinics Hospital Medicine School, University of São Paulo, Brazil(HC/FMRP-USP) under protocol number 3350/2013, and registered in the Brazilian Clinical Trials Registry (ReBEC) under protocol number (RBR-2RZT5C).This study meets the requirements established by the Consolidated Standards of Reporting Trials (CONSORT).
Sample and setting
The sample will be composed of subjects over 50 years of age, both sexes, who present chronic dizziness, as their main complaint, of peripheral vestibular origin. These patients are admitted to Othorhinolaryngology Outpatient Clinic of the Department of Ophtalmology, Othorhinolaryngology and Head and Neck Surgery, HC/FMRP-USP, in the city of Ribeirão Preto, state of São Paulo, Brazil, where they are diagnosed and receive care. Patients with chronic dizziness of vestibular origin, i.e., with symptoms developed for at least three months of the first episode with no compensation,11 are referred to the Department of Speech Therapy, where they are given conventional vestibular rehabilitation (VR).Patients who are not responding positively to rehabilitation for a period equal to or greater than three months will be screened and their names will be listed so they can be contacted by the investigators.
Recruitment
After the screening in the Department of Speech Therapy, the first contact with the participant will be carried out by the investigator in charge with the aim of ensuring the eligibility criteria of the volunteer related to the subject’s participation in the study, with regard to inclusion and exclusion criteria, as well as ethical aspects. At this time, the investigator in charge will conduct a prior registration based on the volunteer's personal information, such as address and phone number, which will be transcribed on an identification list. Patients who accept and meet the eligibility criteria will be directed to Physiotherapy Section at HC/FMRP-USP and will be scheduled to go to the Laboratory of Assessment and Rehabilitation of Equilibrium (LARE) for an interview, to sign the Free and Informed Consent form, and to start the functional and cognitive assessments. Those who do not meet the eligibility criteria or do not agree to participate in the protocol will receive guidelines with regard to the importance of balance rehabilitation offered by physiotherapeutic care.
Inclusion and exclusion criteria
The eligibility criteria comprise subjects of both sexes; over 50 years of age; diagnosed with chronic dizziness and decreased postural balance of peripheral vestibular origin; unspecified lightheadedness or sensations of dizziness with peripheral etiology; daily, weekly and monthly episodes for at least six months; whose symptoms of vertigo, dizziness, and lack of postural stability of vestibular origin do not respond positively to conventional VR, which includes the reorganization of the vestibulo-ocular reflex (VOR).
The eligible patients will be those who do not respond positively to VR, which means that present otoneurologic symptoms for over six months after the beginning of symptoms and show no clinical improvement. The diagnosis of chronic dizziness of peripheral vestibular origin will be confirmed at the Othorhinolaryngology Outpatient Clinic of the Department of Ophtalmology, Othorhinolaryngology and Head and Neck Surgery, HC/FMRP-USP.
The exclusion criteria for the research include the following: patients who receive drugs (benzodiazepines and anticonvulsants) which affect balance or calcium channel blockers (cinnarizine and flunarizine);show restricted mobility, visual restriction and cognitive constraint which preclude the development of the assessments and intervention proposed; or present systemic diseases without any drug control.
The Mini-mental state examination (MMSE) includes the evaluation of orientation to time and place, registration of words, attention and calculation, recall, language and visio-constructive praxis.3 MMSE will be used to exclude those who present cognitive impairments based on the achieve scores: equal to or lower than 15 for illiterate individuals, scores equal to or lower than 22 for those who have 1 to 11 years of schooling, and scores equal to or lower than 27 for those who have over 11 years of schooling.12
Randomization
In order to ensure the process of randomization and a blind experiment for this study, the investigator in charge will choose some assistants: assistant 1 will be in charge of the sample randomization (by allotment), who is not involved in the clinical trial, while the assistant 2 will be in charge of the process of therapeutic intervention.
After selection of the assistants for this study, assistant 1 will contact the volunteers in person after the initial assessment, carried out by the investigator in charge, and will give each volunteer a card with a number code, as well as advising the patients to bring their identification cards to all events related to the research. The randomization will be achieved gradually as the volunteers are screened, wherein each block of six volunteers, the same will be drawn and assigned to the three groups in this study, assuring homogeneity in distribution between groups.
The evaluator (investigator in charge) will always identify participants with these codes, at the first assessment, after 12 intervention sessions and at the three-month follow-up. During this period, the volunteers will be advised to maintain their daily activities; however they will not be allowed to participate in any type of intervention for balance rehabilitation or in a vestibular procedure, except for special cases.
Blinding
As it is a study based on intervention through supervised exercises, neither therapist nor patient can be blind. Therefore, this study will be blind for the evaluator (investigator in charge), who will carry out all assessments in accordance with the study schedule. The process of blinding of intervention will be ensured by assistant 2, who will be a professional Physiotherapist authorized to work at the Physiotherapy Section of the Integrated Center for Rehabilitation of State Hospital at Ribeirão Preto, only for research purposes. This person will be in charge of all interventions and will neither contact the evaluator (investigator in charge) nor get information about the prior or subsequent evaluations. Moreover, assistant 2 will advise the participants regarding the management of groups related to the proprioceptive exercise protocol with and without the anchor system, as well as which protocol should be used for each participant.
After the intervention program, assistant 1 will schedule the final assessments with the evaluator (investigator in charge), and will be in charge of scheduling the assessments after the three-month follow-up (Figure 1).
Outcome measures
The Primary Outcomes will be the balance assessments in the quiet standing and dynamic posture, through Balance Master® system (Neurocom International, Inc., Clackamas, OR). The upright quiet standing will be performed in three positions, with a randomized sequence: two positions without the anchor system (arm extended along the body and arm with an elbow bent in 90º) and one with the anchor system. In the position with anchors usage, the volunteer will hold the anchors in order to keep the flexible cables extended and the weight in constant contact with the ground. The dynamic assessments will follow the pre-established protocols provided by the equipment: walk test, sit-to-stand transfer sensitized test and stability limit, with the activities sequence randomized.
With the aim of avoiding inadequate postural strategies for the stability limit test, which is not intuitive,13 all participants will be acquainted with the platform for 10 minutes, as well as with visual feedback and guidelines regarding strategies for center of pressure (COP) displacement during the movements to be done. The following variables will be assessed:14
Walk test: Step Width (SW), lateral distance in centimeters (cm) between the left and right foot in successive passes; Step Length (SL), longitudinal distance from the left heel and the right in successive passes (cm); Speed of walk (SW), speed in centimeters per second (cm/sec) during the walk; Final Postural Oscillation (FPO), average speed in degrees per second (º/sec), the anteroposterior component of the displacement of the center of gravity within 5 seconds after the end of the gait.
Sit-to-stand transfer sensitized test: The variable measured in this test is the speed of postural sway in degrees per second (°/sec), based on determination of the deviations of the COP on the supporting surface with respect to time.
Limit of stability (LOS): Movement latency (ML), average in seconds(s) from the visual stimulation to the beginning of the movement; Movement velocity (MV), measured in displacement degrees per second (º/sec); average speed of Body Center of Mass (BCOM) measured in (º/s); Endpoint excursion (EPE), defined as the higher BCOM displacement in the first sustained movement in each direction, measured in displacement percentage under the possible maximum displacement deemed to be 100%; Maximum excursion (ME), defined as the higher displacement achieved throughout the testing, in each direction, measured in percentage; Directional control of movement (CM), defined as an individual’s ability to keep the axis of movement parallel to the targeted axis measured in percentage.
With the aim of ensuring the safety of all assessments on the platform, the investigator in charge and an assistant will stay close to the participant in order to avoid any fall episodes and will observe the movement strategies carried out by the volunteers during the tests.15
The clinical functional tests will be performed for the functional performance, balance, agility and gait evaluations, such as:
Timed Up and Go (TUG): The volunteer get up in an ordinary chair with four fixed legs and backrest column, walks 3 meters, turn, walk back, and sit down. The test will be conducted three times the average of the three trials was calculated;16
Sitting-rising test (SRT): will be measured by the time required (seconds) to stand up and sit down five times as quickly as possible;17
Dynamic Gait Index (DGI): consists of eight tasks of walking: 1) march performed by the individual in its normal speed, 2) acceleration and deceleration, 3) movement of head rotation, 4) movement of head flexion-extension, 5) axial rotation of the body, 6) go over the obstacle, 7) go around obstacles and 8) go up and down stairs. The score in each task is based on concepts of dysfunction: absent (three points), minimum (two points), moderate (one point) or severe (zero). The total score ranges from 0 to 24, the highest score being related to better performance.13
Mini-BESTest (MBT): an instrument that assesses the subsystems involved in balance control. It is divided into four categories: biomechanical factors, postural control, sensory orientation, and gait. According to the instructions of the instrument, the items are scored from zero to two, totaling 32 points, in which as nearest the zero will be worse postural control voluntary.18
For the assessment of the impact of dizziness and lack of body stability on the performance of activities in subjects with vestibular disorders will be considered:
The Dizziness Handicap Inventory will be used for assessment of dizziness, consists of 25 questions, with seven items that assess physical health totaling 28 points; 9 items that assess the emotional aspects totaling 36 points and 9 items for the functional capacity of the volunteer, totaling 36 points. The score is classified into four grades: no disability (0-25 points), mild disability (26 to 50 points), moderate disability (51-75 points) and severe disability (76-100 points).19
The instrument Vestibular Disorders Activities of Daily Living Scale (VADL) will be administrated for assessing the impact of body imbalance and dizziness, vestibular disorders of individuals in performing daily activities, which contemplates three dimensions: functional (12 activities), locomotion (9 activities) and instrumental activities of daily living (7 activities) contemplating the total of 28 activities, which the varies score (0-10 points) as the patient's perception of their performance and independence in activity compared to time period to before the development of vestibular disorder.20
The Secondary Outcomes will be specific assessments of cognitive variables such as:
The Clock Drawing Test (CDT),21 consists in asking the volunteer to draw the numbers and watch hands, marking the time of 11:10, not to mention the need for pointers. The score ranges from zero (complete inability to represent the clock) to five (perfect clock, without error), working semantic memory and executive function of the frontal lobe.
The verbal fluency test (VFT), aims to evaluate the semantic memory, in which volunteers mentioned names of animals over a period of 60 s. To attribute a score for this activity will be adopted the following criteria: volunteers with low education (less than nine years) assigned the cut off score will be nine animals per minute and for those with high school volunteers (over nine years), the cutoff score will be 13 animals per minute, according to previous studies.22
For the assessment of visual processing speed and motor function, attention, and mental flexibility, will be adopted the Trail and Making Test B (TMB). The researcher guides the volunteer to draw a trail connecting numbers (sequence 1-13) and letters (A through L sequence) previously exposed in circles randomly. The score is obtained by measuring the erroneous sequences (alphabetic and numeric) and the execution time of each task. The application of this test will follow the guidelines in Physician's Guide to Assessing and counseling older drivers.23
Sample size calculation
To calculate the sample size, GraphPad Statmate software was used, considering the variable of speed of movement of the stability limit in through the Balance MasterÒ system as a primary outcome, which resulted in: sample size=17 (per group), power=0.9, α error=0.05. Taking into consideration possible withdrawals during the study, 60 volunteers will be recruited and they will be divided into three groups:G1 (n=20) intervention protocol: Proprioceptive exercises without the anchor system; G2 (n=20) intervention protocol with anchor:Proprioceptive exercises with anchor system; and G3 (n=20) Control without any kind of intervention.
Interventions
Subsequently, the proprioceptive exercises will be carried out, adapted from Lustosa et al.24 & Lord et al.25 which will be demonstrated and supervised by the assistant in charge of the intervention.
The exercises will be applied to groups G1 and G2, but only group 2 will use the anchor system. The exercises will be performed in sitting and standing positions:
Sitting position
Progression: 1st and 2nd weeks, 3x20 sec; 3rd and 4th weeks, 3x40 sec; 5th and 6th weeks, 3x60 sec.
Standing posture (Figure 2)
Progression: 1st and 2nd weeks, 3x10 sec; 3rd and 4th weeks, 3x20 sec; 5th and 6th weeks, 3x30 sec.
Progression: 1st and 2nd weeks, distributing the body weight back and forth; 3rd and 4th weeks, adding motion to distributing the body weight to the diagonal sides; 5th and 6th weeks, adding motion to make circles with the body.
Progression: 1st and 2nd weeks, 1x 15 repetitions; 3rd and 4th weeks, 2x 12 repetitions; 5th and 6th weeks, 3x10 repetitions.
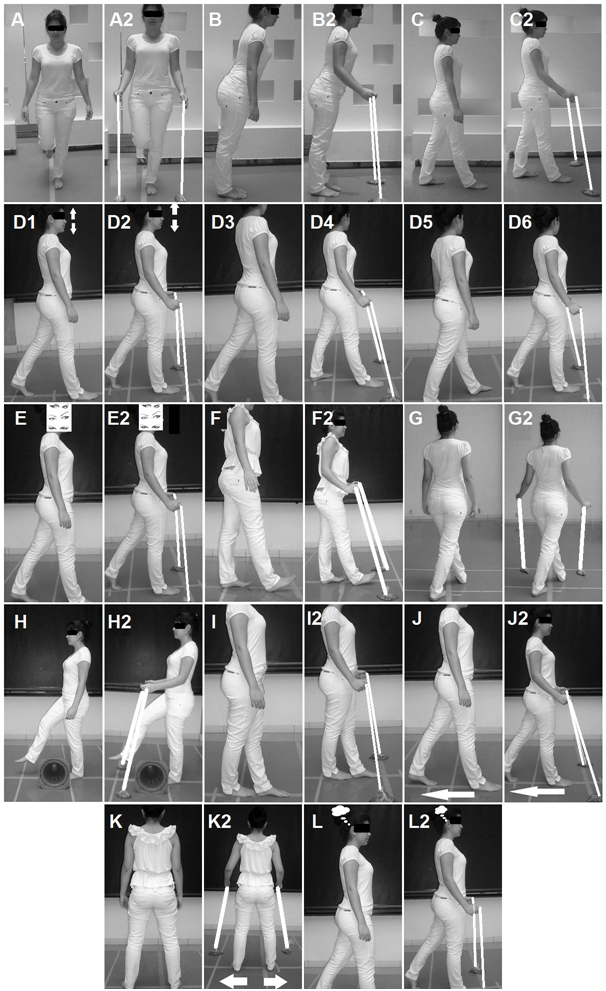
Figure 2 Samples of exercises: A) Single leg stance; A2, Single leg stance with anchor; B) Simulate Stability Limit Test; B2, Simulate Stability Limit Test with anchor; C) Anteroposterior trunk sway, C2, Anteroposterior trunk sway with anchor; D) In stable ground, walk in a straight line previously delimited on the ground D1, cervical flexion and extension, D2 cervical flexion and extension with anchor; D3. Adding motion to cervical lateral rotations, D4, Adding motion to cervical lateral rotations with anchor; D5, Adding motion to trunk movement with dissociation of shoulder girdle and pelvic girdle, D6, Adding motion to trunk movement with dissociation of shoulder girdle and pelvic girdle with anchor; E) Gait with ocular deviation and fixed head, E2, Gait with ocular deviation and fixed head with anchor; F) Gait with calcaneus support only, F2, Gait with calcaneus support only with anchor; G) Gait in diagonal, G2, Gait in diagonal with anchor; H) Gait stepping over obstacles, H2, Gait stepping over obstacles with anchor; I) Tandem gait, I2, Tandem gait with anchor; J) Gait with posterior displacement, J2, Gait with posterior displacement with anchor; K) Lateral gait, K2, Lateral gait with anchor; L) Gait with cognitive activity, L2, Gait with cognitive activity with anchor.
Gait
The gait exercises should be performed at a 6-meter distance, where the progression for all groups will be: 1st and 2nd weeks, 1x 15 repetitions; 3rd and 4th weeks, 2x 12 repetitions; 5th and 6th weeks, 3x10 repetitions.
Statistical analysis
For the purpose of analyzing the effects of intervention among the groups, will be evaluated through intent-to-treat analyses,26 considering the participation of 85% of sessions (10 sessions) of the subjects allocated , in order to ensure both the randomization process and a balanced distribution of prognostic factors in the compared groups. Categorical and quantitative data will be tabulated and presented in spreadsheets to do a descriptive analysis (mean, standard deviation, minimum, maximum, median and percentage) for the quantitative variables. After that, a Shapiro-Wilk test of normality will be conducted to check the normal distribution of data, which will justify the choice of the best test for the analyses of statistical inference. All statistical analyses will be conducted with the SPSS for Windows, version 11.0 (SPSS Inc.) and a significance level of 5% (p≤0.05) will be adopted.
The symptoms associated with chronic peripheral vestibulopathy impact negatively on the independence and quality of life of these subjects.27 Also, the literature has shown that the better the postural balance, the better the functional capacity of subjects with chronic peripheral vestibular disorders, and the bigger the impairment of functional capacity, the bigger the risk of falls in these subjects.28
Therefore, the pursuit of new and efficacious therapies with the aim of improving the clinical situation of these patients is extremely important and relevant, especially for those patients diagnosed with chronic peripheral vestibulopathy who present vertigo symptoms and dizziness of vestibular origin that are persistent and recurrent and do not respond well to conventional vestibular rehabilitation. By increasing the stimulation of the somatosensory system with environmental exploration through anchors in the use of the haptic system, the anchor system is likely to increase the importance of this sensorial system to compensate the impairment of vestibular system.
Therefore, if we deem the increase of life expectancy a worldwide phenomenon, and we can also expect increases in chronic degenerative diseases, otoneurologic symptoms as age advances, lack of postural stability as the main consequence of balance disorders, reduced functional capacity and increases in fall episodes, it becomes necessary to elaborate low-cost strategies with easy clinical application in order to reduce the costs related to healthcare services for this increasing population that is at risk of developing falls and their consequences.
ARC and DCCA were responsible for the conception and design of the study. DCCA makes a important critical review of intellectual content. All authors form those responsible for the production, review and final approval for publication.
The authors of this manuscript have no competing interests.

©2018 Coelho, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.