MOJ
eISSN: 2574-8130


red cell distribution width, biomarker, elderly, risk factors
RDW: Red Cell Distribution Width
For many years, clinical researchers have spent a lot of time and funding obtaining prognostic factors with a good predictive value for acute and chronic disorders. However, such markers are not available word wide, and its determination adds an extra cost to the patient and health institutions.1 Red blood cell distribution width (RDW) is a parameter of circulating erythrocytes measured by hematology analyzer. It is calculated automatically and it is expressed as a percentage. A normal RDW is 11.5 to 14.5%. This parameter has been typically used in hematology to diagnose microcytic anemia. However current studies have shown that RDW played a key role in acute and chronic diseases through various potential physiological and pathological manifestations.2,3 In fact, in the last 10 years, clinical studies have shown strong statistical and independent associations between higher RDW and several diseases such as cardiovascular diseases, diabetes, cancer, pneumonia and gastrointestinal disorders, as well as other acute or chronic conditions, which in turn are present in older adults.4,5 As RDW is an inexpensive and routine biomarker, with high reproducibility and is widely available in all hematological analyzers, efforts to establish its clinical role could be very valuable for morbidity and mortality risk stratification in older adults.
RDW, Morbidity and mortality in older adults
RDW has been historically useful to diagnose anemia-related disorders such as iron, folate and vitamin B12 deficiencies, sickle cell-β-thalassemia and immune hemolytic anemia.6,7 For many years, the RDW was only considered useful for hematological diseases. However, Felker et al. in an elegant paper, demonstrated a close relationship between increased RDW and poor prognosis in patients with cardiovascular diseases, irrespective of their anemia status.8 Subsequently, this biomarker began to be explored more deeply in various cardiovascular diseases such as acute or chronic heart failure, acute myocardial infarction, atrial fibrillation, among others. These studies reported a high predictive value related to cardiovascular morbidity and mortality.9-11 More interestingly, several reports expanded this notion by assessing the relationship of RDW with other acute or chronic diseases, such as diabetes, cancer, kidney diseases, liver failure and other pathologies, as shown in Figure 1. For example, the use of the RDW in the diagnosis of malignant tumors (i.e. endometrial, lung, colon and liver cancers) has recently attracted much attention.12 A high RDW has been positively correlated with the stage of several cancers, including the number of metastatic tissues, tumor diameter, and over expression of established cancer biomarkers.12 Likewise, current reports have showed higher RDW levels in patients with neurological or psychiatric disorders such as migraine, depression and anxiety.13,14
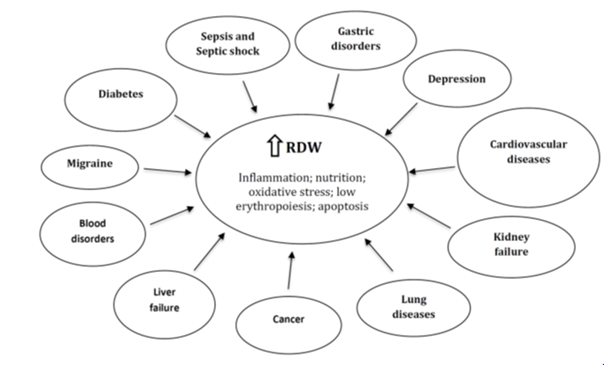
An important question: How does the RDW increase?
Although different studies have demonstrated that RDW is a robust and independent predictor of morbidity and mortality compared with another established risk biomarkers, the mechanism explaining why a high RDW value is associated with acute or chronic diseases remains unknown. It has been proposed that inflammation and oxidative stress are the main events that could alter erythrocyte homeostasis and therefore to induce a RDW increase.2,3 In this regard, inflammation inhibits bone marrow function and iron metabolism.2,3,16 Moreover, proinflammatory cytokines inhibits erythropoietin-induced erythrocyte maturation and proliferation, which is reflected partly by a RDW increase.15 Lippi et al., reported that the RDW was positively correlated with the erythrocyte sedimentation rate and high-sensitivity C-reactive protein level, indicating that the RDW reflects the inflammatory state of the body.16 In addition, metabolic abnormalities such as shortening of telomere length, poor nutritional status (deficiencies of iron, folic acid, and vitamin B12), dyslipidemia, hypertension, erythrocyte death or alteration of erythropoietin function might contribute to RDW increase.17,18 All these alterations are common in older people; thus, we could hypothesize that RDW is a “master” blood biomarker reflecting the general health status in the elderly.
Considering the economic, technical and clinical advantages, the measurement of RDW alone or combined with other stablished clinical biomarkers could be very useful for improving the risk stratification of morbidity and mortality of many diseases. However unraveling the detailed mechanisms associated with higher levels of RDW is necessary for the future acceptance of this parameter within the medical and scientific community as a general and useful marker.
None.
The authors declare no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.