MOJ
eISSN: 2573-2919


Short Communication Volume 9 Issue 1
1Universidad Politécnica Metropolitana de Puebla, Puebla, México
2Universidad Tecnológica de Izúcar de Matamoros, Izúcar de Matamoros, Puebla, México
3Benemérita Universidad Autónoma de Puebla, Puebla, México
Correspondence: Navarro-Frómeta Amado Enrique, Universidad Tecnológica de Izúcar de Matamoros, Prolongación Reforma 168, Izúcar de Matamoros, Puebla, México, Tel +52- 2431064994
Received: January 30, 2024 | Published: February 14, 2024
Citation: Horta-Valerdi GM, Crespo-Barrera PM, Mendoza-Hernández JC, et al. What do we breathe near contaminated water bodies? MOJ Eco Environ Sci. 2024;9(1):24-27. DOI: 10.15406/mojes.2024.09.00303
Samples of total suspended particles were taken at points located in the vicinity of two polluted rivers of Puebla, México, an affluent of the Atoyac River (UPMP), the Nexapa River (ICATEP), a point at some distance from the Nexapa River (UTIM) and one point far from this stream (sCarlos). 1 L water samples were taken from the two streams (aAtoyac and Nexapa). Sampling and extraction of organic contaminants was performed according to USEPA method TO13A and analyzed by gas chromatography/mass spectrometry. In addition, DNA was extracted from the samples and sequenced. In previous work, a group of semi-volatile emerging contaminants were analyzed and in this work, 8 compounds with lower volatility were selected. Water concentrations of the studied compounds were much higher for aAtoyac than for Nexapa. The results obtained allow us to establish that the contaminants present in the water are aerosolized and therefore can affect the population that is exposed to aerosols from heavily polluted rivers with decreasing concentration in the order UPMP>ICATEP>UTIM>sCarlos with a decrease in their relative concentrations with distance from the water body. We conclude that proximity to heavily contaminated bodies of water implies serious risks to human health. It is worth mentioning that the obtained results represent only a first glance of the studied problem. A deeper evaluation obviously require more sampling and varying the distances from the rivers to determine time-space variations of the pollutant’s concentrations in aerosols and bioaerosols near polluted water bodies.
Keywords: polluted rivers, aerosolization, organic pollutants, bacteria
PM, particulate matter; EC, emerging contaminants; VOCs, volatile organic compounds; TSP, total suspended particles; DNA, deoxyribonucleic acid
Almost the entire world population breathes air that does not comply with the limits stipulated by the World Health Organization, with suspended particles or atmospheric particulate matter (PM) being around 62% of the deaths attributable to air pollution in 2019, in addition to many other illness.1–3 To the above, considering a comprehensive approach to pollution, we must add the environmental and health issues related to water pollution, urbanization with the problems associated with vehicle emissions, and in general any anthropic action that negatively affects ecosystems and consequently the causes of diseases.4 Furthermore, proximity to contaminated sites or living near where polluting activities such as livestock farming are carried out entails health risks.5,6
The first thing you notice when approaching a highly polluted waterbody are the odor and color characteristic of the type and degree of pollution,7–9 which is even related to an empirical approach to the evaluation of its quality and the willingness to use it, which even affects the health of the population close to it.10,11 This is due to the passage of contaminants from the aqueous phase to the gas phase and the formation of aerosols. This aerosolization process resembles that which occurs in wastewater treatment plants due to the formation and breaking of bubbles that give rise to fine droplets loaded with organic contaminants, including volatile organic compounds and emerging contaminants.12–14 Therefore, it is expected that contaminated water bodies under the action of air, and turbulence, promote the formation of contaminated aerosols and bioaerosols, especially in urban environments.15–17 The relationship between organic and microbiological contaminants present in seawater and marine aerosol at the mouth of the Tijuana River has been reported.18 Once in the atmosphere and associated with PM, emerging contaminants (ECs) or pollutants of emerging concern affect human health through various pathways.19
The airborne microbiome is important to human health as many microorganisms have a wide distribution worldwide. Although, studies generally focus on bacteria, fungi, viruses, and archaea. Considering that exposed areas of the human body include the skin, oral, nasal, and respiratory surfaces, it is evident that contact with airborne microorganisms is continuous. One of the main groups of study corresponds to bacteria, which are widely distributed in the atmosphere and range between 10 and 107 cells m3 estimated by methods using culture media, molecular biology, and rapid estimation methods. Much of the study of bacteria corresponds to Gram-negative bacteria, which can produce endotoxins released into the environment and can cause various infections and toxicity when present in large concentrations. Moreover, bacterial biodiversity is extensive, and various dominant bacterial genera have been found, including Streptophyta, Bacillus, Corynebacterium, Pseudomonas, Acinetobacter, Kocuria, Staphylococcus, Sarina, Sphingomonas, Chryseobacterium, Sejongia, Vibrio, Micrococcus, etc.20 Bacteria are typically not found alone but attached to PM, forming nuclei that can survive with nutrients obtained from clouds, water, and particles themselves until their deposition, depending on climatic conditions.20,21 This leads to an increased exposure to microorganisms transported in the bioaerosols a topic that is still underexplored.22 Nowadays, attention is focused on the presence of bacterial and antibiotic-resistant genes, because their dispersion near highly polluted urban wastewater represents a significant threat to human health.23–25
In previous studies, it was shown that many EC, with relatively low volatility, are found in the vicinity of heavily polluted rivers (Crespo-Barrera et al., 2023). Volatile organic compounds (VOCs) deserve special attention due to the role they play in the formation of precursors that worsen air quality and lead to the formation of ozone.26 Considering also that polluted rivers behave like arteries that generate and transport considerable masses of aerosols and bioaerosols,27 this work presents the results of the determination of some VOCs in the atmospheric particulate in the vicinity of heavily contaminated rivers, as well as the bacteria identified therein.
The study was carried out 10 m from two water streams in the State of Puebla, a heavily contaminated tributary of the Atoyac River (water sample identified in green as eAtoyac in Figure 1D) and the Nexapa River (water sample identified in green as Nexapa in Figure 1B) near the city of Izúcar de Matamoros, from which 1L water samples were taken by procedures normalized. In both cases, samples of the total suspended particles (TSP) were taken using a TE-1000 polyurethane cartridge sampler (TISCH Environmental), following a procedure following the USEPA TO13A method for cleaning crystalware, conditioning the polyurethane foam and filter, sampling, treatment and extraction of the samples obtained. Additionally, samples were taken of TSP at the Technological University of Izúcar (air sample identified as UTIM in Figure 1B), further away from the Nexapa River and as a control sample away from contaminated water bodies, on a hill near a hot springs resort (air sample identified as SCarlos in Figure 1C).
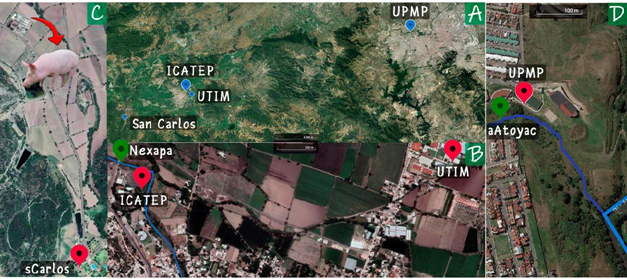
Figure 1 A) General distribution of the sampling sites, B) water sample of the Nexapa River (in green) and the air sample near this stream (ICATEP; in red), and the control sample far away from the river (UTIM). C) Control sample far away from polluted water bodies (sCarlos) and D) water sample of a highly polluted tributary of the Atoyac river (in green, aAtoyac) and its corresponding air sample (in red, UPMP).
The organic extracts were analyzed by gas chromatography coupled with mass spectrometry. The description of the method used is described elsewhere (Crespo-Barrera et al., 2023). The quantification of the contaminants was carried out using the ratio between the peak area of the compound and the peak area of the internal standard in each sample, normalizing the area ratios to 1 m3 and 1 L for air and water samples, respectively.
The microbiological study of bioaerosols was only carried out on the UPMP, ICATEP, and UTIM air samples.
To study the metagenomics of the air filters, the following was carried out: DNA was extracted from the sampling filters with lysozyme and with the specification of the Macherey Nagel Genomic DNA extraction kit; 1% agarose gels were carried out to corroborate the presence of DNA. Quantification was performed with a Nanodrop microvolume spectrophotometer. The DNA extracts were sent to an external laboratory for sequencing.
It is known that different alcohols, aldehydes, ketones, and sulfur compounds, among other low molecular mass compounds, are easily found in the emissions from sites with strong fecal contamination. For this work, eight of the first eluted compounds, unambiguously identified, were selected for the analysis: Cyclohexanol (CHnol); Cyclohexanone (CHone), Methyl 2-(methylthio) acetate (M2MTA); Ethanol2butoxi (E2B); Benzaldehyde (BzA); Phenol (Phen); Dimethyl trisulfide (DMTS) and 2,5-dithiahexane (25DTH). Indol (IND), Skatol (SKA), and coprostanol (COP) were also selected for the study considering the strong fecal pollution in the studied streams. Figure 2 shows the corresponding area relations of the selected compounds.
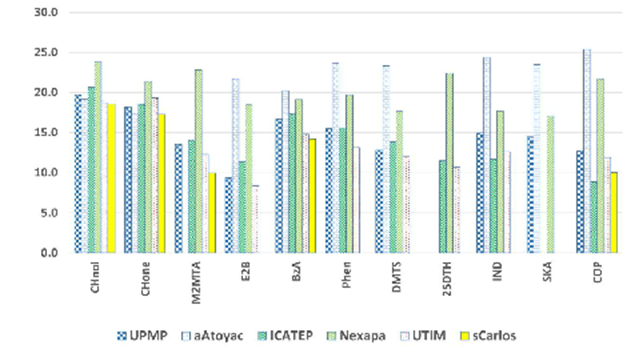
Figure 2 Areas of the selected pollutants relative to the internal standard: Cyclohexanol (CHnol), Cyclohexanone (CHone), Methyl 2-(methylthio)acetate (M2MTA), Ethanol2butoxi (E2B), Benzaldehyde (BzA), Phenol (Phen), Dimethyl trisulfide (DMTS), 2,5-dithiahexane (25DTH), Indol (IND), Skatol (SKA) and coprostanol (COP).
It was unexpected that some of these VOCs were found at the control point but considering that 1.4 km from this point there is a pig farm (Figure 1C), there is a plausible explanation for their presence. Then it is not surprising that CHnol and CHone appear in all the sampled sites since they are solvents for various uses, including as coformulants of pesticides, and these compounds have been detected among the VOCs in emissions from livestock and poultry facilities. Both compounds present toxicity to humans through contact or inhalation.28–30
It can be seen that for several of the contaminants, as expected, there is an appreciable decrease in their relative concentrations in the sequence UPMP>ICATEP>UTIM, related to the distance from the river and the load pollution of the different streams, and it must be considered that 3.4 km from the University, there is a chicken farm and that the institution is surrounded by irrigation canals that lead the water of the Nexapa River.
Regarding the microbiological study of bioaerosols, as expected, the DNA concentration found in the filters varied significantly. A higher amount of DNA was found in the sample corresponding to the Atoyac River area (UPMP and eAtoyac samples), while the lowest was observed in the UTIM area (Crespo-Barrera et al., 2023).
The sequencing of samples obtained from the three sampling points aligns with the isolated genetic material. The UTIM sample presents the lowest number of bacterial genera (Figure 3) while UPMP (Atoyac river area) exhibits a higher number of bacterial genera (Figure 5). In the bioaerosols from the UTIM sample, the predominant genera in the sample are Bacillus (64%), followed by Firmicutes, and to a lesser extent, Proteobacteria, which includes pathogenic bacteria, with Pseudomonas being the main genus.
In the bioaerosol sample from the ICATEP, near Nexapa River (Figure 4), the percentage of the Bacillus genus is lower (43%), although there is a significant increase in the presence of pathogenic Proteobacteria, including Enterobacteria, and an increase in Pseudomonas (19%).
In the UPMP sample (Atoyac River area, Figure 5), Bacillus decreases compared to the less contaminated area to 41%. However, there is an increase in microbial biodiversity with the presence of Lactobacillales, Pseudomonas, and Proteobacteria, including the genera Enterobacter, Pantoea, Salmonella, and Citrobacter.
From the analysis of the microbial biodiversity of airborne microorganisms present in the three samples, it was found that the Atoyac area has the highest microbial biodiversity. There are 6 bacterial genera in the UPMP sample that are not present in UTIM or ICATEP. It also shares another 6 genera that are present in UTIM and ICATEP, of which there are 3 genera in common with UTIM and ICATEP, 2 genera shared with ICATEP, and only 1 genus shared with UTIM. The area with the lowest microbial biodiversity is UTIM, which presented 6 bacterial genera, however only one genus is present in UTIM but not in the other two samples. UTIM has one genus in common with ICATEP and UPMP. For the Nexapa area, 7 bacterial genera were identified, where it shares 2 genera with UPMP and has 2 genera that are not present in the other sampling zones (Figure 6).
Measuring microbial biodiversity in airborne microorganisms is not an easy task, but it is of utmost importance to dedicate efforts to this aspect. Understanding how biodiversity increases and distributes through various generation sources and different climates could help in reducing microbial biodiversity. By controlling the initial and crucial aspects of its generation, it could contribute to a decrease in respiratory diseases.7,20 The DNA extraction results align with microbial biodiversity (Figure 4) and correspond well enough to chromatographic analysis (Figure 2). In all three microbiological analyses, it is observed that contamination decreases in the order Atoyac sampling site > Nexapa sampling site > UTIM, which follows the same trend exhibited by the chromatographic analysis, in which sCarlos (the control sample) is the less polluted one.
Comparing the three bioaerosol samples, it is evident that the most contaminated sample is from the Atoyac River (UPMP), representing a greater risk to human health in the nearby areas. The presence of a considerable number of colony-forming units and microbial DNA in the analyzed PM samples indicate a health risk, especially for those living near riverbanks and those who spend extended periods in such areas. This aspect needs attention and should be studied using the methodology outlined by the World Health Organization for determining such risks.
None.
The experimental work was carried out with the financial support of the Council of Science and Technology of the State of Puebla.
The authors declare no conflict of interest in writing the manuscript.

©2024 Horta-Valerdi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.