MOJ
eISSN: 2573-2919


Review Article Volume 5 Issue 3
Department of Chemistry, Faculty of Science and Technology, Universitas Airlangga, Komplek Kampus C UNAIR, Jl. Mulyorejo, Surabaya, 60115 Indonesia
Correspondence: Nanik Siti Aminah, Assoc. Professor, Department of Chemistry, Faculty of Science and Technology, Universitas Airlangga, Komplek Kampus C UNAIR, Jl. Mulyorejo, Surabaya, 60115 Indonesia, Tel +62-31-5936501, Fax +62-31-5936502
Received: June 09, 2020 | Published: June 22, 2020
Citation: Rosyda M, Aminah NS, Kristanti AN. Various ester derivatives from esterification reaction of secondary metabolite compounds: a review. MOJ Eco Environ Sci. 2020;5(3):141-151. DOI: 10.15406/mojes.2020.05.00187
Secondary metabolite compounds have a very diverse structure that is widely used as a source of new drug discovery because they have a variety of bioactivity. But in its development, there are several problems related to these compounds including low bioavailability, low solubility and instability in the metabolic process. Modification of the structure of secondary metabolites is used to answer all these problems. One of the processed was by synthesising the ester derivative compounds through the chemical and enzymatic esterification reaction. Esters derivatives of secondary metabolite compounds can increase the diversity of structures, allow for increased biological activity and even new biological activity of these compounds. This review will discuss various processes of modification of the structure of secondary metabolite compounds through chemical and enzymatic esterification reactions that have been reported from 1994-2019.
Keywords: synthesis, esterification reaction, secondary metabolite compounds, ester derivatives, enzymatic
Natural product compounds were obtained from various organisms. Natural product compound consists of primary and secondary metabolites. Primary metabolites are compounds produced by living things and are essential in the process of cell metabolism for cell survival. Primary metabolites are consisting of carbohydrates, fats, proteins and nucleic acids. Secondary metabolites are compounds that are only found in certain organisms or groups of organisms and are found in nature in limited quantities. Secondary metabolite compounds consist of terpenoids, steroids, phenyl propanoids, flavonoids, stylbenoids and alkaloids.1 Natural product compounds have extraordinary potential to be developed into high value products both as pharmaceutical products, nutritional products, functional foods, and cosmetics.2
Secondary metabolites have a very diverse structure that is widely used as a source of new drug discovery because it has a variety of bioactivity.3 Even though there are many secondary metabolite compounds that have the potential to become new drug candidates, there are still some problems related to these compounds. These problems include bioavailability and low solubility and instability in the metabolic process.4–6 Therefore, many structural modifications are made to natural product compounds to enrich new structures, allowing an increase their biological activity and even new biological activity of these compounds, so that the discovery of new drugs is growing. One of the processed of structural modification is by synthesising its ester through the esterification reaction.
Esterification reaction is a reaction between carboxylic acid compounds and their derivatives with alcohol to form ester compounds. Esterification reaction of secondary metabolite compounds can be done through esterification reactions by chemical or enzymatic reactions. Esterification reaction using a chemical catalyst is used because the process is relatively easy, but the selectivity is low and requires expensive costs. In recent years, the reaction approach using enzyme catalysts is in great demand. The enzymatic approach is preferred because of its high catalytic efficiency, enables easier and efficient separation of compounds, mild reaction conditions, require lower costs and environmentally friendly process.7–10 Based on several studies have reported that the lipase enzyme has been widely used as a catalyst for ester synthesis. Lipase (EC 3.1.1.3) functions to catalyze various reactions such as, esterification, hydrolysis and transesterification.11,12 Some lipase enzymes used for catalysts in esterification reagents include Lipozyme TLIM, Lipozyme 435, Novozym 435, Porcine pancreatic lipase.13–19
So far there has not been review that discusses the various processes of the esterification reaction of secondary metabolite compounds. Therefore, this article will discuss the process of modification of the structure of secondary metabolite compounds through chemical and enzymatic esterification reactions that have been reported from 1994-2019. This review aims to provide various references regarding the synthesis of esters derived from secondary metabolites.
Ester derivatives of cinnamic acid
Cinnamic acid is one of the phenolic compounds of the phenyl propanoid group. Several researchers have reported the synthesis of esters from cinnamic acid (1) through enzymatic esterification reactions in solvent free or organic solvent systems. Some of these esters include ethyl cinnamate (2), butyl cinnamate (3), benzyl cinnamate (4) and cinnamic acid L-ascorbate (5).5,16,27,28 Ethyl cinnamate was synthesized from esterification reaction with Lipozyme TLIM catalyst. The reaction mixture containing cinnamic acid and ethanol in isooctane was incubated in incubator shaker at 40˚C at 170 rpm and produced high yield of 86%.27 Several researches were synthesized ethyl cinnamate (2), but the yield is very low. Synthesis of ethyl cinnamate (2) was also catalyzed by Novozym 435 enzyme through esterification reaction of cinnamic acid with ethanol. Yield of ethyl cinnamate is very low (2%) and requires a long-time reaction (7 days).13 Other researchers have synthesized ethyl cinnamate (2) using Porcine lipase as a biocatalyst.14 Ethyl cinnamate (2) was synthesized by enzymatic reactions using Novozym 435 lipase and the yield of 35.2%.16
Butyl cinnamate (3) was synthesized from esterification reaction of cinnamic acid (1) with the Novozym 435 lipase catalyst. The reaction mixture containing cinnamic acid (1), butanol in hexane and incubated in a temperature-controlled incubator shaker at 30˚C at 250rpm.16 Benzyl cinnamate (4) was synthesized from esterification reaction of cinnamic acid (1) with benzyl alcohol and catalyzed by Lipozyme TLIM in several organic solvents such as acetone, trichloromethane, methylbenzene, and isooctane. The reaction mixture was incubated in an incubator shaker at 150rpm at 40˚C. Based on this reaction, isooctane media produced the highest yield.28 L-ascorbyl cinnamate (5) was synthesized through esterification reaction which is catalyzed by Lipozyme TLIM and Novozym 435 enzyme in various solvents including methanol, ethanol, acetonitrile, and acetone. Acetone and acetonitrile were reported as the most suitable media. Lipase catalyzed using Lipozyme TLIM dan Novozym 435 gave a maximum yield of 99%.15 Addition of Ph-TiO2 to the enzymatic esterification reaction for formation of cinnamyl ester was reported to increase catalytic efficiency in the reaction (Figure 1) (Figure 2) (Table 1).29
|
Compounds |
R reagent |
Enzyme |
Yield (%) |
Reference |
|
(2) |
EtOH |
Lipozyme TLIM |
86 |
Wang et al.,18 |
|
(3) |
Buthanol |
Novozym 435 |
46 |
Jakovetic et al.,16 |
|
(4) |
Benzyl alcohol |
Lipozyme TLIM |
79.88 |
Wang et al.,27 |
|
(5) |
L-ascorbic acid |
Lipozyme TLIM Novozym 435 |
99 |
Zhang,15 |
Table 1 Reagents, conditions and yield in esterification reactions of cinnamic acid
Eugenol (6) is one of the phenolic compounds from phenylpropanoid group. Eugenol (6) was isolated from various plants including Eugenia caryophyllata, Pimenta racemosa, Cinnamomum verum, Caryophyllus aromaticus, and Syzygium aromaticum.17,22 Several ester compounds from eugenol were synthesized through chemical and enzymatic esterification. Eugenol derivative esters compounds that were reported their synthesis process, include eugenyl acetate or 4-allyl-2-methoxyphenyl acetate (7), eugenyl caprylate (8), and eugenyl benzoate (9) (Figure 3).
Eugenyl acetate [4-allyl-2-methoxyphenyl acetate] (7) can be synthesized through reaction of eugenol (6) with acetic anhydride and pyridine to gave yield of 63.20%.22 Eugenyl acetate (7) was also synthesized from reaction of eugenol (6) with acetic anhydride and NaOAc to produce eugenyl acetat (7) of 63.65%.30 In addition, the synthesis of eugenyl acetate (7) was carried out through an esterification reaction between acetic anhydride and NaOH and produced 86.87% eugenyl acetate product.23
Several researchers have also reported the synthesis of eugenyl acetate (7) through an enzymatic esterification reaction using Lipozyme TLIM lipase and Novozym 435 as a catalyst and acetic anhydride as an acyl donor.17,19 Eugenyl acetate is also produced by esterification of eugenol (6) and acetic anhydride in SC-CO2 using two lipase enzymes as catalysts namely Lipozyme 435 and Novozym 435. Eugenyl acetate was also produced by esterification of eugenol (6) and acetic anhydride in SC-CO2 using two lipase enzymes as catalysts namely Lipozyme 435 and Novozym 435. The Novozym 435 gave higher yield than Lipozyme 435.31
Eugenyl caprylate (8) was produced through the esterification of eugenol (6) with caprylic acid using Lipozyme TLIM as a catalyst. Eugenol (6), caprylic acid and Lipozyme TLIM enzyme were reacted into the n-hexane solvent at 65oC and stirred at 250rpm for 259 minutes. This condition was optimum to give good yield of 72%.33 Eugenyl benzoate (9) was synthesized by an enzymatic reaction between benzoic acid and eugenol (6) using S. aureus lipase [24] and Rhizomucormiehei lipase as a biocatalyst.34,35 The reaction mixture was incubated and shaken with heating. The yield of eugenol benzoate products through enzymatic esterification reaction using S. aureus lipase and Rhizomucormiehi were 56.13% and 65% (Figure 4) (Table 2).
|
Compounds |
R reagent |
Catalyst/Enzyme |
T (˚C) |
Yield (%) |
Reference |
|
(7) |
Acetic anhydride |
Pyridine |
Rt |
63.2 |
Carasso et al.,22 |
|
NaOAc |
80 |
63.65 |
Manoppo,30 |
||
|
NaOH |
70 80 |
86.87 |
Riswanto,23 |
||
|
Lipozyme TLIM |
55 |
93 |
Machado et al.,19 |
||
|
Novozym 435 |
60 |
99.87 |
Vanin et al.,17 |
||
|
(8) |
Caprylic acid |
Lipozyme TLIM |
65 |
72 |
Chaibakhsh et al.,33 |
|
(9) |
Benzoic Acid |
S. aureus lipase |
41 |
75 |
Horchani et al.,24 |
|
|
|
Rhizomucormiehi |
50-65 |
56-66 |
Table 2 Reagents, conditions and yield in esterification reactions of cinnamic acid
Quercetin (10) is one of the phenolic compounds from flavonoid group. Several ester compounds from Quercetin (10) were synthesized through chemical and enzymatic esterification reaction. 3,3’,4’,7-tetraacetate quercetin (11) have been synthesized through the esterification reaction between quercetin (10) with acetic anhydride and using pyridine as a catalyst at room temperature for three hours. This process produced 75% of esters.36 3,3’,5,7-tetraacetate quercetin (12) was produced by the esterification reaction of acetic anhydride and pyridine at room temperature for 24 hours and gave yield of 70%.38 3,3’,4’,7-tetraethylcarbamate quercetin (13) was synthesized from the mixture of quercetin, ethylbromo acetate, K2CO3, KI, and acetone at 55˚C for 6 hours.5
3,3’,4’,7-tetraisobutyrate quercetin (14), 3,3’,4’,7-tetrapivalate quercetin (15), and 3,3’,4’,7-tetrabenzoate quercetin (16) were obtained from the reaction of quercetin (10) and several acid anhydrides with pyridine as a catalyst at various conditions. 3,3’,4’,7-tetraisobutyrate quercetin (14) was produced from the reaction of 1mol of quercetin (10), 12.6mol of Isobutyric anhydride and pyridine at 70˚C for 2 hours and produced good yield of 96%. 3,3’,4’,7-tetrapivalate quercetin (15) was synthesized through the reaction between 1mol of quercetin (10) with 24mol of pivalate anhydride and pyridine at 65˚C for 45 minutes and produced ester product (15) of 85%. While 3,3’,4’,7-tetrabenzoate quercetin (16) was synthesized from the reaction of 1mol of quercetin (10) with 12.6mol of benzoyl chloride and pyridine at 70˚C for 2 hours and produced 72% ester product.36
Several researches have also reported the process of the synthesis of quercetin pentaasil compounds. 3,3’,4’,5,7-pentaacetate quercetin (17) was synthesized from the reaction between quercetin (10) with acetic anhydride and pyridine as catalysts in various conditions, including heated at temperature of 55˚C for 6 hours,4 at 65˚C for 5 hours with yield of 79%,36 at room temperature for 24 hours25 and at 80˚C for 5 days.37 3,5,7,3’,4’-pentaaisobirat quercetin (18), 3,5,7,3’,4’-pentapivalate quercetin (19), and 3,5,7,3’,4’- quercetin pentabenzoate (20) were obtained by the esterification reaction between quercetin (10) with some acid anhydrides and pyridine as catalyst at various conditions. 3,5,7,3’,4’-pentaisobutyric quercetin (18) was produced from the reaction between 3 mol of quercetin (10) with 30 mol of isobutyric anhydride and pyridine at 65˚C for 2 hours and produced good yield of 97%. 3,5,7,3’,4’-pentapivalate quercetin (19) was produced through the reaction of 1 mol of quercetin (10) with 30 mol of pivaloyl chloride and pyridine at 65˚C for 2 hours and produced of 87% ester. While 3,5,7,3’,4’-pentabenzoate quercetin (20) was synthesized by the esterification reaction of 1 mol of quercetin (10) with 30 mol of benzoyl chloride and pyridine at room temperature for 3 hours and gave good yield of 98% (Figure 5) (Table 3).36
|
Compounds |
R1 |
R2 |
R3 |
R4 |
R5 |
|
(11) |
COCH3 |
H |
COCH3 |
COCH3 |
COCH3 |
|
(12) |
COCH3 |
COCH3 |
COCH3 |
COCH3 |
H |
|
(13) |
COOC2H5 |
H |
COOC2H5 |
COOC2H5 |
COOC2H5 |
|
(14) |
COCH(CH3)2 |
H |
COCH(CH3)2 |
COCH(CH3)2 |
COCH(CH3)2 |
|
(15) |
COC(CH3)3 |
H |
COC(CH3)3 |
COC(CH3)3 |
COC(CH3)3 |
|
(16) |
COC6H5 |
H |
COC6H5 |
COC6H5 |
COC6H5 |
|
(17) |
COCH3 |
COCH3 |
COCH3 |
COCH3 |
COCH3 |
|
(18) |
COCH(CH3)2 |
COCH(CH3)2 |
COCH(CH3)2 |
COCH(CH3)2 |
COCH(CH3)2 |
|
(19) |
COC(CH3)3 |
COC(CH3)3 |
COC(CH3)3 |
COC(CH3)3 |
COC(CH3)3 |
|
(20) |
COC6H5 |
COC6H5 |
COC6H5 |
COC6H5 |
COC6H5 |
Figure 5 Esterification reaction of quercetin.
|
Compounds |
R reagent |
Catalyst/Enzyme |
T (˚C) |
Yield (%) |
Reference |
|
(11) |
Acetic anhydride |
Pyridine |
Rt |
75,00 |
Mattarei et al.,36 |
|
(12) |
Acetic anhydride |
Pyridine |
Rt |
70,00 |
Ortega et al.,38 |
|
(13) |
Ethylbromo acetate |
K2CO3 |
55 |
95,00 |
Hu et al.,5 |
|
(14) |
Isobutyric anhydride |
Pyridine |
70 |
96,00 |
Mattarei et al.,36 |
|
(15) |
Pivalic anhydride |
Pyridine |
65 |
85,00 |
Mattarei et al.,36 |
|
(16) |
Benzoyl chloride |
Pyridine |
70 |
72,00 |
Mattarei et al.,36 |
|
(17) |
Acetic anhydride |
Pyridine |
65 |
79,00 |
Mattarei et al.,36 |
|
(18) |
Isobutyric anhydride |
Pyridine |
65 |
97,00 |
Mattarei et al.,36 |
|
(19) |
Pivaloyl chloride |
Pyridine |
65 |
87,00 |
Mattarei et al.,36 |
|
(20) |
Benzoyl chloride |
Pyridine |
65 |
98,00 |
Mattarei et al.,36 |
Table 3 Reagents, conditions and yield in esterification reactions of quercetin
Resveratrol (21) is a polyphenol compound derived from stilbenoid which is contained in a variety of plants including grapes and nuts.39 Several ester derivatives of resveratrol (21) have been synthesized through the esterification reaction through chemical and enzymatic reactions. Ester of resveratrol that has been reported in the synthesis process include 4’-acetate resveratrol (22), 4’-isobutyrate resveratrol (23), 4’-butyrate resveratrol (24), 4’-pivalate resveratrol (25), 4’-benzoate resveratrol (26), 3-acetate resveratrol (27), 3,4’,5-triacetate resveratrol (28), 4’-octanoate resveratrol (29) 3,5-dioctanoate resveratrol (30) and 3,4’,5-trioctanoate resveratrol.
Selective resveratrol esterification reaction at position 4' such as 4’-acetate resveratrol (22), 4’-isobutyrate resveratrol (23), 4’-butyrate resveratrol (24), 4’-pivalate resveratrol (25), and 4'-benzoate resveratrol 26 is carried out under thermodynamic conditions using acid anhydrides and NaH base catalysts.40 The 4’-acetate resveratrol (22) was also synthesized through the reaction between acetic anhydride and various base catalysts and solvents. Each reaction condition includes acetic anhydride, K2CO3 base, and EtOH solvent at room temperature; acetic anhydride, base InCl3, and MeCN solvent at room temperature and acetic anhydride, Et3N base, and DMSO at 65˚C. The reaction used Et3N and DMSO solvents gave a maximum yield of 4’-acetate resveratrol (22) of 47.00%.26
The mixture of 4-acetate resveratrol (22) and 3-acetate resveratrol (27) was synthesized by reaction between resveratrol and acetic anhydride with some base catalysts such as pyridine, NaOH, K3CO3 and NaH and some lewis base catalysts such as FeCl3, NiCl2, InCl3, ErCl3, TiCl3.4H2O, and TiO2 and used several solvents such as acetic acid, THF, MeCN, CH2Cl2, EtOH, H2O and DMSO. The reaction used of NaH base, and THF solvents give 4'-acetate resveratrol (22) and 3-acetate resveratrol (27) products most at 47.00% and 9.40%.40 3,4’,5-triacetate resveratrol (28) was synthesized through the reaction between resveratrol and acetyl chloride, Et3N, and acetone for 16 hours at room temperature. This reaction produced ester of 78%. 3,4’,5-triacetate resveratrol (28) was also synthesized from the reaction of resveratrol with acetic anhydride, pyridine and acetic acid solvent and the reaction between resveratrol with acetyl chloride and pyridine and CH2Cl2 solvent at room temperature.41 4’-octanoate resveratrol (29), 3,5-dioctanoate resveratrol (30) and 3,4’,5-trioctanoate resveratrol (31) were mixed products from the reaction between octanoyl chloride as an acyl donor with Et3N and ethyl acetate solvents at 25oC for 12 hours.6
Several researchers also reported the processes of resveratrol ester synthesis through enzymatic reactions. 4-acetate resveratrol (22) and 3,5,4’-triacetate resveratrol (28) were obtained through an enzymatic esterification reaction. 4-acetate resveratrol (22) was synthesized by added the enzyme Candida antartica to the resveratrol solution (dissolved in t-amyl alcohol solvent) containing vinyl acetate. 3,5,4’-triacetate resveratrol (28) was synthesized by added the enzyme Candida antartica into resveratrol solution (dissolved in BuOH solvent) containing acetic anhydride and pyridine. The mixture was incubated at 40˚C at 400rpm (Figure 6) (Table 4).42
|
Compounds |
R1 |
R2 |
R3 |
|
(22) |
H |
H |
COCH3 |
|
(23) |
H |
H |
COCH(CH3)2 |
|
(24) |
H |
H |
COC3H7 |
|
(25) |
H |
H |
COC(CH3)3 |
|
(26) |
H |
H |
COPh |
|
(27) |
H |
COCH3 |
H |
|
(28) |
COCH3 |
COCH3 |
COCH3 |
|
(29) |
H |
H |
COC8H17 |
|
(30) |
H |
COC8H17 |
COC8H17 |
|
(31) |
COC8H17 |
COC8H17 |
COC8H17 |
Figure 6 Esterification reaction of resveratrol.
|
Compounds |
R Reagent |
Catalyst/Enzyme |
T (˚C) |
Yield (%) |
Reference |
|
(22) |
Acetic anhydride |
Et3N |
65 |
47,00 |
Lepart et al.,26 |
|
Acetic anhydride |
NaH |
65 |
40,00 |
Acerson & Andrus,40 |
|
|
Vinil asetat |
Candida antartica |
40,00 |
Nicolosi et al.,42 |
||
|
(23) |
Isobutyric anhydride |
NaH |
65 |
43,00 |
Acerson & Andrus,40 |
|
(24) |
Anhidrida butirat |
NaH |
65 |
46,00 |
Acerson & Andrus,40 |
|
(25) |
Anhidrida pivalat |
NaH |
65 |
58,00 |
Acerson & Andrus,40 |
|
(26) |
Benzoil klorida |
K2CO3 |
65 |
32,00 |
Acerson & Andrus,40 |
|
(27) |
Acetic anhydride |
NaH |
65 |
9,40 |
Acerson & Andrus,40 |
|
Acetic anhydride |
Candida antartica |
40 |
30,00 |
Nicolosi et al.,42 |
|
|
(28) |
Acetyl chloride |
Et3N |
rt |
78,00 |
Jing,41 |
|
(29) |
Octanoyl chloride |
Et3N |
25 |
*ND |
Hu et al.,6 |
|
(30) |
Octanoyl chloride |
Et3N |
25 |
*ND |
Hu et al.,6 |
|
(31) |
Octanoyl chloride |
Et3N |
25 |
*ND |
Hu et al.,6 |
Table 4 Reagents, conditions and yield in esterification reactions of resveratrol
*ND, no data
Several kaurenoic esters namely methylkaur-16-en-19-oate (33), butylkaur-16-en-19-oate (34), Benzylkaur-16-en-19-oate (35), 4-chlorobenzylkaur-16-en-19-oat (36), 4-bromobenzilkaur-16-en-19-oat (37), 4-florobenzilkaur-16-en-19-oat (38), 4-nitrobenzilkaur-16-en-19-oat (39) have been synthesized from kaurenoic acid. These compounds were obtained through the esterification reaction of kaurenoic acid with suitable alkyl halides namely CH3I for (33), C4H9Br for (34), PhCH2Br for (35), 4-ClPhCH2Br for (36), 4-BrPhCH2Br for (37), 4-FPhCH2Br for (38), and 4-NO2PhCH2Br for (39), in KOH-acetone at 25˚C (Figure 7) (Figure 8) (Table 5).43
Compounds |
(33) |
(34) |
(35) |
(36) |
(37) |
(38) |
(39) |
Yield (%) |
89 |
71 |
91 |
48 |
74 |
37 |
21 |
Table 5 Yields of kaurenoic esters compound
Derivative ester of betilinic acid (40), namely bentulinyl hexanoate (41) has been synthesized from the reaction between betulinic Acid with hexanoyl chloride and DMAP (4-dimethylamino pyridine) as catalyst in CH2Cl2. The reaction mixture was reacted at room temperature for 24-48 hours. This process gave yield of 94% (Figure 9).44
Terpenoid ester compounds have also been synthesized through an esterification reaction with an enzymatic catalyst. Geranyl acetate and geranil benzoate ester compounds have been synthesized from geraniol [3,7-dimethylocta-2,6-dien-1-ol (42)] namely 3,7-dimethylocta-2,6-dien-1-yl acetate (43) and 3,7-dimethylocta-2,6-dien-1-yl benzoate (44). 3,7-dimethylocta-2,6-dien-1-yl acetate (43) was synthesized by the reaction between 3,7-dimethylocta-2,6-dien-1-ol (42) and vinyl acetate in toluene with the enzyme Pseudomonas cepacia lipase as a catalyst at 55˚C for 3 hours. This reaction produced 99% of ester.20 Whereas 3,7-dimethylocta-2,6-dien-1-yl benzoate (44) was obtained by the esterification reaction of 3,7-dimethylocta-2,6-dien-1-ol (42) with benzoic acid, diethylazodicarboniclicate (DED) and triphenylphosphite (PPh3) in THF at 0˚C. This process produced 95% ester product (Figure 10).45
Other terpene ester derivatives have also been synthesized. 2-(4-methylcyclohex-3-en-1-yl) propan-2-yl acetate (46) was synthesized from 2-(4-methylcyclohex-3-en-1-yl)propan-2-ol (45) and acetic anhydride in supercritical carbon dioxide (SC-CO2) with enzymatic catalysis using C. rugosa lipase. The reaction mixture of (45) compound and acetic anhydride is added to the reactor, followed by addition of C. rugosa lipase. After that CO2 is pumped into the reactor and the esterification reaction is carried out at 10MPa and 50˚C for 1.5 hours with at 250rpm. This process produced 53% of ester.46 3,7-dimethylox-2-en-1-yl acetate (48) was synthesized from 3,7-dimethylox-2-en-1-ol (47) with vinyl acetate in n-hexane solvent. The reaction was catalyzed by Pseudomonas sp. The reaction mixture was incubated at 30˚C at 200rpm for 24 hours.47 The geranil benzoate ester namely (1S)-2-isopropyl-5-methylcyclohexane-1-yl benzoate (50) was produced through the Mitsonabu esterification reaction of (1R)-2-isopropyl-5-methylcyclohexane-1-ol (51) with benzoic acid, diethylazodicarbonicate (DED) and triphenylphosphite (PPh3) in THF at 0˚C. This process gave yield of 95% (Figure 11) (Figure 12).45
The process of synthesis of ester compounds from citronellol was also reported. The citronellyl acetate (52) was synthesized by the reaction of citronellol (51) with vinyl acetate in n-hexane solvent and catalyzed by Pseudomonas sp. The reaction mixture was incubated at 30˚C at 200rpm for 24 hours. This process gave ester product of 80.2%.47 The citronellyl acetate (52) was also synthesized by the enzymatic reaction using the Carica papaya lipase as a catalyst to gave yield of 99%.48 The citronellyl acetate (52) was also obtained from the reaction mixture of dry n-hexane, citronellol (51), acetic acid and catalyzed by Candida antarctica lipase. The reaction mixture was incubated at 30˚C and 200rpm to give an ester product of 98%.49 The citronellyl malonate (53) was synthesized using by the C. rugose enzyme as a catalyst. Malonic acid was reacted with citronellol (51) and inserted into a reactor that contains the lipase enzyme at 310K. This process produced yield of 98% (Figure 13).50
Ester derivative of menthol namely mentyl propionate (55), was synthesized through the enzymatic reaction using the lipase enzyme AY-30 from Candida cylindracea. Menthol (54) was dissolved in n-hexane and reacted with propionic anhydride. The mixture was incubated for 48 hours at 30˚C. Yield of ester product is 30% (Figure 14).51
The esterification of terpenes was also used ionic liquids as catalysts in reactions. Geraniol acetate (43), terpineol acetate (46) citronellol acetate (52), and mirceneol acetate (57) was synthesized using Sulfonic Functionalized Ionic Liquids (SFILs) as a catalyst. Terpenol acetate was obtained by reacted each terpenol using acetic anhydride with catalyst (SFILs). The sterification by SFILs catalysts gave high yield more than 90% (Figure 15).52
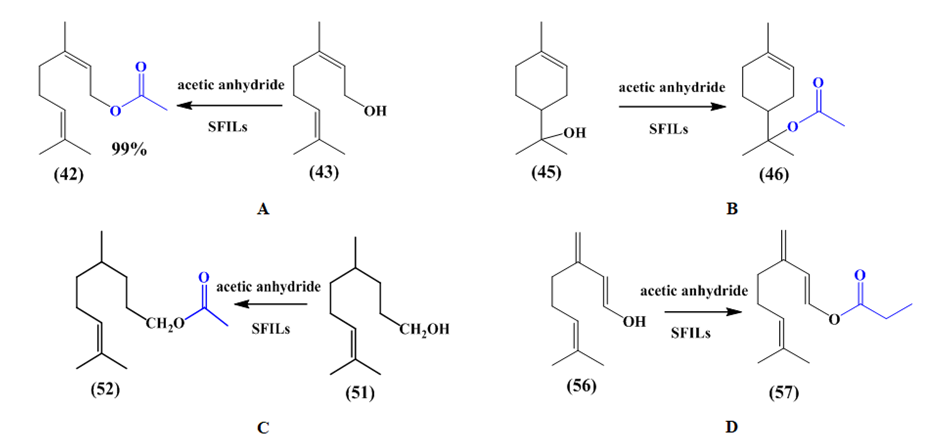
Figure 15 Formation Reaction of Esters: A. geraniol acetate, B. terpineol acetate, C. citronellol acetate and D. mirceneol acetate.
Several esters of terpenoids alcohol with phenolic acid were synthesized including 1,3,3-trimethylbicyclo-[2.2.1]-heptan-2-yl-3,4-dihydroxybenzoate (58), 1,3,3-trimethylbicyclo-[2.2.1]-heptan-2-yl-3,4,5-trihydroxybenzoate (59), 1,3,3-trimethylbicyclo-[2.2.1]-heptan-2-yl-3-(4-hydroxy-3-methoxyphenyl)acrylate (60), 1,3,3-trimethylbicyclo-[2.2.1]-heptan-2-yl-3-(3,4-dihydroxyphenyl)acrylate (61). All of these esters were synthesized by estrification reaction of monoterpenic fencol with various phenolic acids (protokatekuat acid for (58) compound, gallic acid for (59) compound, caffeic acid for (60) compound, and ferulic acids for (61) compound) and N,N-Dicyclohexylcarbodiimide (DCC) in THF for 24 hours at room temperature (Figure 16) (Figure 17).53
Compounds |
N |
R1 |
R2 |
R3 |
58 |
0 |
OH |
OH |
H |
59 |
0 |
OH |
OH |
OH |
60 |
1 |
OH |
OH |
H |
61 |
1 |
OH |
OMe |
H |
Figure 17 Esterification reaction of terpenes alcohol.
Derivative esters of steroid
Steroid derivative esters compound 5α-Androstan-17β-(6-methylheptan-2-yl)-30β-yl benzoate (63) was synthesized from the steroid compound 5α-Androstan-17β- (6-methylheptan-2-il)-30β-ol (62) through the esterification reaction of Mitsonabu by reacted steroids and benzoic acid with diethylazodicarbonicate (DED) and triphenylphosphite (PPh3) in THF at 0oC. This process gave yield of 65%.45 5α-Androstan-30β, 17β-diol-3-acetate (65), 5-Androsten-3β, 17β-diol-3-acetate (67) and 5-Androsten-3β,6β-diol-17-on-3- acetate (69) more obtained from the reaction of 5α-Androstan-30β,17β-diol (64) (for (65) compound), 5-Androsten-3β,17β-diol (66), (for (67) compound), and 5-Androsten-3β,6β-diol-17-on (68) (for (65) compound) with vinyl acetate. Each reaction mixture was dissolved in a THF solvent and the reaction was catalyzed by C.a.B. lipase. The reaction mixture was shaken at 45˚C for 40 hours.54
Cipoterone (70) derivative esters have been synthesized including cipoteronyl propionate (71) and cipoteronyl benzoate (72). Ciprotonil propionate (71) was synthesized through the esterification reaction between cipoteronil (70) with propionyl chloride and N-Bromosuccinimide in CH2Cl2 for 12 hours. Cipoteronyl benzoate (72) was synthesized from the reaction of cipoteron (70) with benzoic acid using H2SO4 and acetonitrile for 20 hours (Figure 18) (Figure 19).55
Esterification reaction in secondary metabolites is used to produce compounds that have different biological activities that are even better than the original compounds.20,21 The compound eugenol acetate (7) has been reported have better anticancer activity compared to eugenol. Eugenol acetate compounds more actively inhibit the growth of KB cancer cells (oral squamous carcinoma cells) and DU-145 (androgeninsensitive prostate cancer cells) with IC50 values of eugenol acetate and eugenol in KB cells respectively 21.26×10-6 mol.L-1 and 28.48×10-6 mol.L-1 and DU-145 cells respectively 21.5×10-6 mol.L-1 and 30.39×10-6 mol.L-1.22 The addition of acetyl groups to eugenol was reported to increase the anti-inflammatory activity of eugenol because it can increase the selectivity of inhibiting prostaglandin formation in the COX-2 (cyclooxygenase 2) pathway.23,32 The eugenol acetate (7) was also reported to have potential as a compound that can be used in larvicidal formulations because the toxicity of eugenyl acetate against Aedes aegypti larvae is higher than eugenol with an LC50 value 0.102mg/mL.19 Therefore, eugenyl benzoate (9) has the potential as an antioxidant drug because it has been reported to have more antioxidant activity than the BHT used as a control. The antioxidant activity of eugenol benzoate is higher compared to eugenol with IC50 value for each compound that is 18.2lg/ml and 20.20lg/ml.24
Several ester derivatives of queccetin were reported for their biological activity. 3,3’,4’,7-tetraacetate quercetin was reported to have a different biological activity that is potentially antiviral to Human Respiratory Syncytial Virus (hRSV) which is a virus that causes pneumonia. This biological activity is not owned by quercetin. 3,3’,4’,7-tetraacetate quercetin interacts with M2-1 protein from hRSV on the binding side of M2-1 RNA. Therefore, these compounds have the potential as inhibitors of hRSV virus replication.37 Compound of 3,3',5,7-tetraacetate quercetin was reported to have anti-inflammatory activity because it is able to inhibit the production of Nitrogen Oxide (NO) which acts as a proinflammatory mediator synthesized by inducible nitric oxide synthase (iNOS).38
3,3’,4’,7-tetraethylcarbamate quercetin (13) was reported to have higher permeability than quercetin so that they are able to more easily cross human epithelial cells (CaCo-2 cells). The transport rate of 3,3’,4’,7-tetraethylcarbamat quercetin (13) is significantly higher than the quercetin of 5.23×10-6cm/s, whereas the transport rate of quercetin is (10) 2.82×10−6cm/s.5 3,3,4’,5,7-pentaacetate quercetin (17) was reported to have higher bioavailability compared to quercetin (10) to human epithelial cells (MDCK-1 and MDCK-2).4 This compound was also reported to have anticancer activity against glioma cells whereas the quercetin did not have anticancer properties against glioma cells.25 In addition, this compound was reported to have antiviral activity against Human Respiratory Syncytial Virus.37
The biological activity of resveratrol ester derivatives was also reported. 4’-acetate resveratrol (22) was reported to have antiaging activity. This compound significantly increased more than 3.3 times the gene expression of antiaging factors including extracellular matrix proteins (elastin and collagen III, IV), SIRT 1, MT1H (metalloproteinases), FBN1 (skin aging biomarkers fibrillin), LAMB1 (laminin), PCNA (proliferating cell nuclear antigen), and skin growth factors (HBEGF, IGF1, NGF, and TGF). The 4'-acetate resveratrol compound (22) also decreased the expression of inflammatory and aging skin molecular genes (COX-2, IL-1, IL-6, IL-8). Based on these data, 4'-acetate resveratrol (22) has the potential for the prevention and treatment of skin aging.26
The 4'-octanoate resveratrol (29), 3,5-dioctanoate resveratrol (30) and 3,4 ', 5-trioctanoate resveratrol (31) observed the stability of the gastrointestinal digestive system model and compared with resveratrol. The results reported that the bounded caprilate subtituents were relatively stable without hydrolysis in the mouth and gastric phase. However, in the intestine, the caprilate subtituents are not hydrolyzed and release resveratrol. This shows that caprilate esters from resveratrol can be absorbed by the intestinal lumen in the form of free resveratrol.6
Methylkaur-16-en-19-oat (33), Butylkaur-16-en-19-oat (34), Benzylkaur-16-en-19-oat (35), 4-Chlorobenzylkaur-16- en-19-oat (36), 4-Bromobenzilkaur-16-en-19-oat (37), 4-Fluorobenzylkaur-16-en-19-oat (38), 4-Nitrobenzylkaur-16-en- 19-oat (39) were reported to have lower antifungal activity than the original compound namely kaurenoic acid. Bentulinil hexanoate compound (41) was reported to have antitumor activity against cells SF-295, HCT-116, PC-3 and HL-60 but the activity of bentulinyl hexanoate (41) on each tumor cell is still lower compared to bentulinc Acid (40). Esterification of bentulinc Acid causes a decrease in antitumor (Table 6).44
|
No. |
Compounds |
Bioactivity |
Reference |
|
(7) |
eugenyl acetate |
Anticancer & antiinflamatory |
Carrasco et al.,22; Riswanto,23 & Saraphanchotiwitthaya, et al.,32 |
|
(9) |
eugenyl benzoate |
antioxidant |
Horchani et al.,24 |
|
(11) |
3,3’,4’,7-tetraacetate quercetin |
antiviral |
Guimaraes et al.,37 |
|
(12) |
3,3’,5,7-tetraacetate quercetin |
antiinflamasi |
Ortega et al.,38 |
|
(13) |
3,3’,4’,7-tetraethylcarbamate quercetin |
high bioavailability |
Hu et al.,5 |
|
(22) |
4’-acetate resveratrol |
antiaging |
Lepart et al.,26 |
|
(29) |
4’-octanoate resveratrol |
hydrolyzed in the intestine |
Nicolosi et al.,42 |
|
(30) |
3,5-dioctanoate resveratrol |
hydrolyzed in the intestine |
Nicolosi et al.,42 |
|
(31) |
3,4’,5-trioctanoate resveratrol |
hydrolyzed in the intestine |
|
|
(33) |
Methylkaur-16-en-19-oate |
antifungal |
Boecka et al.,43 |
|
(34) |
Butylkaur-16-en-19-oate |
antifungal |
|
|
(35) |
Benzylkaur-16-en-19-oate |
antifungal |
|
|
(36) |
4-chlorobenzylkaur-16-en-19-oate |
antifungal |
|
|
(37) |
4-bromobenzylkaur-16-en-19-oate |
antifungal |
|
|
(38) |
4-fluorobenzylkaur-16-en-19-oate |
antifungal |
|
|
(39) |
4-nitrobenzil kaur-16-en-19-oate |
antifungal |
|
|
(41) |
Bentulinyl hexanoate |
antitumor |
Vicktor et al.,44 |
|
(58) |
1,3,3-trimethylbicyclo[2.2.1]heptan-2-yl-3,4-dihydroxybenzoate |
lipoxygenase enzyme inhibitors |
Sadegian et al.,53 |
|
(59) |
1,3,3-trimethylbicyclo [2.2.1]heptan-2-yl-3,4,5-trihydroxybenzoate |
lipoxygenase enzyme inhibitors |
|
|
(60) |
1,3,3-trimethylbicyclo[2.2.1]heptan-2-yl-3-(4-hydroxy-3-methoxyphenyl) acrylate |
inhibitor enzim lipoksigenase |
Sadegian et al.,53 |
|
(38) |
1,3,3-trimethylbicyclo[2.2.1]heptan-2-yl-3-(3,4-dihydroxyphenyl)acrylate |
lipoxygenase enzyme inhibitors |
|
Table 6 Bioactivity of esters derivatives from secondary metabolite compounds
Secondary metabolite compounds have a very diverse structure that is widely used as a source of new drug discovery because they have a variety of bioactivity. These compounds can be modified by esterification reaction with chemical and enzymatic reactions. Esters derivatives of secondary metabolite compounds can increase the diversity of structures, allow for increased biological activity and even new biological activity of these compounds. Based on this review, some of the ester compounds produced have not yet reported for their biological activity. In addition, there has not been much modification of the structure of other secondary metabolite compounds. Therefore,urther research is needed to increase the diversity of structures and enable the discovery of potential new biological activities, so that the discovery of new drugs is developing.
None.
None.
The authors declare there are no conflicts of interest.

©2020 Rosyda, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.