MOJ
eISSN: 2573-2919


Review Article Volume 5 Issue 6
1Research Unit of Analysis and Process Applied to the Environmental, APAE Higher Institute of Applied Sciences and Technology Mahdia, University of Monastir, Tunisia
2LR Biotechnology and Bio-Geo Resources Valorization (LR11ES31), Higher Institute for Biotechnology, University of Manouba, Biotechpole of Sidi Thabet, Tunisia
3Department of Civil, Chemical, Environmental and Materials Engineering (DICAM), University of Bologna, Italy
Correspondence: Dr. Wafa Hassen, Research Unit of Analysis and Process Applied on the Environmental-APAE UR17ES32, Higher Institute of Applied Sciences and Technology Mahdia “ISSAT”, University of Monastir, 5100 Mahdia, Tunisia, Tel 00216-22931899
Received: August 31, 2020 | Published: December 8, 2020
Citation: Hassen W, Cherif H, Souissi Y, et al. Rhizobacteria and their metabolites as a promising green approach for the treatment of pesticide contaminated agricultural soils. MOJ Eco Environ Sci. 2020;5(6):244-254. DOI: 10.15406/mojes.2020.05.00200
Pesticides are employed to control and manage pest populations at tolerable levels. Pesticides are classified especially according to their chemical structure, toxicity, environmental persistence and target organisms. The massive use of these pollutants in addition to their toxic potential seriously threatens ecosystems and humans. For this reason, the development of green bioremediation processes is necessary. The ability of several microorganisms to bioremediate pesticides is mainly based on their biodegradation activity. Though bacteria have been proved to be efficient biodegraders and bioremediators, some fungi and archae could biodegrade recalcitrant pesticides too. The bioremediation of pesticide-contaminated agricultural sites may be optimized by considering the prevalent environmental conditions, the microorganisms that solubilize and degrade the pesticides most effectively, the variables that affect the biodegradation rate and the chemical structure of pesticides. This chapter explores the importance of pesticides as persistent organic pollutants in agricultural soils, particularly in the plants rhizospheric area and further illustrates the recent advances in pesticide microbial bioremediation, with emphasis on the metabolic potential of pseudomonads as a representative model of pesticide-degrading microorganisms.
Keywords: pesticides, agricultural soils, rhizospheric area, toxicity, bioremediation, pseudomonas, enzymes, biosurfactants
Pesticides are world-wide used. Despite the inbalance of use between developed and under developed countries, they still present a major environmental and health concerns. During the last 40years, in modern agriculture, a large number of pesticides were used to control insect pests and to enhance crop production.1,2 Their excessive and unreasonable use causes stress and yield losses in addition to the degradation of soil quality.3,4 Moreover, these chemicals are spread in the environment due to their adhesion to the organic matter in the soil and/or to the plant roots. They highly contaminate soil ecosystems and cause a serious threat to the balance among various groups of microorganisms and the soil components. In the meanwhile, the widespread application of these pesticides disturb the rate and the activity of soil enzymes.5
Pesticides are degraded in the environment, principally by the action of microorganisms or their enzymes.6 After a long-term exposure to these pesticides, some microorganisms can develop resistance and can successfully be used for bioremediation of pesticide-contaminated soils.7,8 Some microbes exhibited an efficient bioremediation potential in the presence of specific pesticides using them as a source of nutrients and energy.9,10
In order to predict the fate of pesticides in soils, it is important to have an understanding of those microbes able to degrade pesticides, their activities and the factors that improve their activity in situ.9,11 Bacteria belonging to the genus Pseudomonas are reported to be metabolically adaptable and able to degrade several organic or inorganic substances such as aromatic hydrocarbons, oil, petroleum products and pesticides.12,13 This genus forms an heterogeneous group of bacteria, present in large numbers in the rhizosphere where they are active in the mineralization of organic matter.14 Moreover, Pseudomonas possesses a variety of diverse catabolic pathways that enable them to metabolize a diverse range of low-molecular-weight compounds.15 Due to the ubiquity and versatility of these Pseudomonads, there is a considerable interest in exploiting these bacteria for diverse agricultural applications. Moreover, these bacteria represent suitable candidates for bioremediation applications. This review gives an overview on the relationship between the pesticide application in agricultural fields and their biodegradation routes. Therefore, the role of rhizospheric Pseudomonas in pesticides biodegradation and their practical application for bioremediation of agriculture contaminated soils will be described.
In modern agriculture, millions of tons of pesticides are applied annually to enhance the production through controlling harmful effects caused by several organisms, including insects, fungi, bacteria, viruses as well as weeds.16 The first use of pesticides in agriculture dates back to antiquity.17 Their development and their extensive use were then concomitant with inorganic chemistry development. The compounds employed are derivatives of inorganic compounds as examples, those based on arsenic, copper, zinc, manganese or nicotine sulfate. Then, from the Second World War, pesticides have benefited from the development of organic chemistry.18 The development of synthetic compounds was also responsible for the rapid expansion of pesticides use from 1940.19 A slowing trend has been observed since the ’80s, partly due to the discovery of more active substances requiring lower tonnages. In addition, this regression was also due to serious ecological and toxicological problems associated with some of these substances. Pesticides are characterized by a large variety of chemical structures, functional groups and diverse activities. That is the reason why their classification is complex.20,21 In the frame of the actual agricultural practices, manufacturers and users classify them according to the target species. We can thus distinguish herbicides, insecticides, fungicides, etc.22 Another classification is made according to the chemical nature of the main active substance. We can cite as examples organochlorines, organophosphates, carbamates, pyrethroids, triazines, etc.23,24 A large variety of pesticides is available on the market. Some examples are presented in Table 1 where specific characteristics such as chemical family, type, structure, toxicological classes and pesticide half-life are reported.21,25,26 Recently, organophosphorus pesticides were classified as the most worldwide used group.27 A large number of organochlorine pesticides were prohibited due to their persistency and accumulation in soil.28 More than 100 organophosphorus pesticides are in use, accounting for about 38% of total pesticide usage.29
In Tunisia, the Pesticide Registration Service announced that the pesticides annual imports average are around 5000 tonnes/year.30 And their use continues to progress (Figure 1); in 2010 it was 3182.1tons, in 2013 it increased to 4113.9tons and finally, in 2016, it is in the order of 6661.9 tons. These quantities of pesticides were imported as active ingredients or as formulations, and were mostly used in agriculture sector (around 95%) in a treated area of approximately 761,000 hectares/year and about 5 kg/hectares. According to the list of pesticides approved in Tunisia by the Pesticide Registration Service,31 732 active ingredients and 849 products were marketed on the local market. Among these pesticides, Fungicides represent 59% of total pesticides imported in Tunisia with 433 products and 359 active ingredients, followed by Herbicides (22%) and Insecticides (17%) (Figure 1).
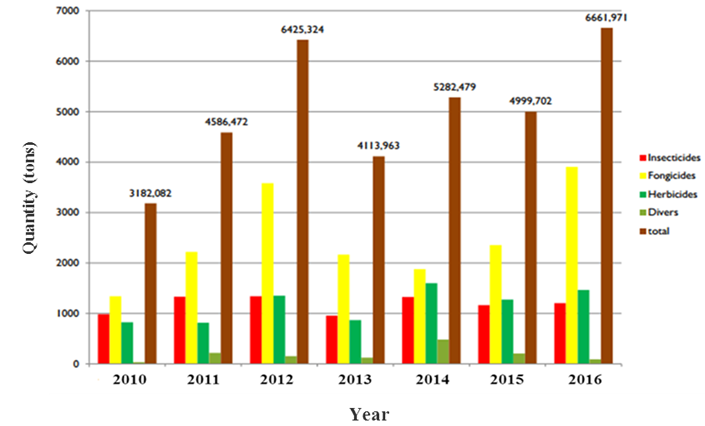
Figure 1 The quantities of pesticides imported in Tunisia.30
Factors influencing the mobility or persistence of pesticides in the rhizosphere
The mobility of pesticides can lead in their redistribution within the application site or the displacement of a part of their quantity of site.32 The persistence of an applied molecule in the rhizosphere is determined by its dissipation, which is the result of all phenomena determining the quantity of substances present in a given compartment at a given moment. It is influenced by many factors, including degradation, sorption and bioavailability as outlined by Arias-Estevez et al.33
Pesticides application method depends on the type of formulation. Usually, they are sprayed on crops and cover the aerial parts of the plants and soil.34 They could also be incorporated into the soil. A large fraction of pesticides seeps into the soil and reaches surface waters and groundwater. An important part of pesticides are found in the atmosphere under the action of various physicochemical or climatic events.. This contamination is also related to the method of application due to pesticide volatilization, wind drift during spraying and wind erosion of soil particles on which pesticides are adsorbed.35 The amounts of chemical products administered vary according to several important factors such as product activity, pesticide persistence, crop type and period of growth. The treatments can be unique to a certain stage of crop growth or fraction. In the latter case, this treatment may range in pre-sowing, pre-planting, pre- or post-emergence. The same culture can receive several treatments all over the year.36,37
The behavior of pesticides in soil is governed by a variety of complex processes.33,38 Depending on the process, the concentration and the form of the molecule vary in the environmental matrices. The pesticide fate in the environment is thus estimated through its half-life referred as DT50. The DT50 is the times taken for the degradation or dissipation of 50% of the amount initially applied.39 DT50 values are usually used to estimate pesticide persistence. Nevertheless, for the same molecule, the observed DT50 values can present a wide range of values illustrating the influence of the variability of climate conditions, soil type and cropping practices.40 The processes that control the transport of pesticide in soil to other environmental compartments are mainly biological and/or chemical degradation, sorption, desorption phenomena,41,42 volatilization, runoff, leaching and plant uptake.43 The relative importance of these processes varies according to the chemical nature of the applied molecules, to soil characteristics and to the climate (Figure 2). The retention routes and the degradation processes of pesticides in soil are the two fundamental phenomena conditioning their environmental fate.33,44
Degradation is essential to decrease the amount of residues in soil. It is controlled by abiotic and biotic factors and involves interactions between the different constituents of the soil, microorganisms and pesticide molecules.45 Abiotic transformations are caused by chemical reactions that are not catalyzed by enzyme systems. The principal abiotic transformations are the reactions of oxidation, reduction, conjugation, hydrolysis, and photoreactions. Biotic degradation is due to the action of various living microorganisms that chemically transform molecules through their enzymatic systems. The main mechanisms of degradation by microorganisms are the direct metabolism, the cometabolism as well as conjugation and condensation reactions.44,46 In addition, retention phenomena play a fundamental role in the fate of pesticides in soils, especially in their persistence.33 They correspond mainly to adsorption, transfer of a compound from the liquid or gaseous phase from the soil to the solid phase, and conversely to desorption. The adsorption is a more or less reversible phenomenon depending on numerous parameters. The content of soil organic matter is a major factor influencing the retention of pesticides on solid soil phase. A positive correlation was observed for the majority of pesticides between retention and content of soil organic matter.47 The pH, clay, soil oxides also influence retention.44
Retention and degradation are closely related.22 Retention decreases the accessibility of compounds to soil microorganisms, reducing their degradability. This coupling retention/degradation allows the determination of substance mobility. The generation of non-extractable pesticide residues are due of two mechanisms that are (I) the formation of covalent bonds between the molecules and the organic material and (II) the sequestration of molecules and/or its degradation products. Organic matter is the key factor of their training. Other factors can influence their formation, such as pH48 and time.49 The formation of these residues is often considered as a dissipation pathway contributing to the elimination of soil pesticides, decreasing the availability to degradation.50 However, this stabilization is not definitive in time and could be the origin of a very diffuse pollution and deferred in time.22,48 The significant presence of residues throughout the food chain is responsible for biodiversity reduction and ecological disruption. This is largely due to bioaccumulation of these substances. Moreover, the multiplication of pesticide treatments has the effect of favoring the appearance of resistance among the targeted organisms (insects, mites,…), which involves the use of increasingly massive doses and more dangerous products. In this case, these products may present associated toxicity risks to biota that were not affected by treatment.51
Pesticide and their derivates toxicity
Pesticides are appearing beneficial in terms of crop quality and productivity, but their harmful side effects were quickly shown. The effect of these pesticides depends of their mode of action as some are more toxic than others, their persistence over time as some degrade much more rapidly than others and their degradation products that could be more toxic and degrades less quickly than the parent molecule.52 Amongst the most alarming problems associated with pesticide application is their possible toxicological impact in the environment. Therefore, their possible incorporation into the food chain affects ecosystems and human beings.53 Thus, 15 to 20% of these chemicals are considered as carcinogens54 and most of them are recognized as endocrine disruptors.55 Another important issue is the effects produced due to the combination of several active substances, which are, generally, superior to individual effects of substances due to the interaction between simultaneously present molecules.56 According to the literature, Odukkathil and Vasudevan51 reported that pesticide degradation products could also be toxic or even more toxic and more mobile than the original molecule. For example, derivatives of Chlorpyrifos and Malathion were at least 100 times more toxic than the parent compounds and diazinon derivatives are 10 times more toxic.57 In addition, when the pesticides are not used within the given time of their efficacy, they become obsolete. They are decomposed into other chemical components, which sometimes become even more toxic than the original pesticides.58 Moreover, many pesticides used in agriculture contain in their formulation adjuvants with active substances that cause serious hazards. We can cite as an example, Dimethoate EC40, containing 400g/L of Dimethoate active ingredients as well as coformulants such as cyclohexanone, xylene and surfactants.59 These components are more dangerous than the active substance and causes serious clinical problem with a fatality rate estimated at of 20.6%.60
Several studies reported that pesticides are cytotoxic, neurotoxic, embryos toxic, mutagenic, teratogenic and carcinogenic.36,61–63 Pesticides can undergo metabolic activation and form electrophilic intermediates able to interact with nucleic acids. Other indirect ways of activation could be observed such as oxidative stress, inhibition of intercellular communication or formation of activated receptors.64 Some pesticides and their degradation products have also been identified as agents that could affect male fertility.62 An exposure to certain substances also could cause a disorder of the immune system.63 Salameh et al.61 reported that chronic exposure to pesticides was associated with symptoms of chronic respiratory diseases in children, especially asthma. Insecticides are reported as the most acutely toxic class of pesticides compared to herbicides and fungicides.36 A toxicology reference center in Tunisia has shown that intoxication by organophosphorus represents 11% of all acute intoxication.65
Many studies focused on pesticide bioremediation due to the reported harmful impact on the environment and associated toxicity effects. Microbial biodegradation was reported as an essential mechanism and an effective biotechnological approach for the dissipation of pesticides in the environment.35,66
Bioremediation is a process used by microorganisms to convert pollutants into less or non-toxic compounds. Microbial pesticide bioremediation can be performed in situ or ex situ.6,67 There are two types of bioremediation: intrinsic bioremediation and enhanced bioremediation.68 The intrinsic bioremediation relies on bacteria that are already present in the soil. Enhanced bioremediation also known as bioaugmentation uses a bacterial consortium selected for its ability to transform contaminants. If the appropriate microorganism is absent or if biodegrading microbial population was reduced due to the toxicity of pesticides, in that case a specific microorganism or an appropriate consortium can be introduced into the soil to enhance the activity of the existing population and their degradation activity.67 These microorganisms could be either natural or genetically modified.69 The adaptive capacities of microorganisms to pesticide are explained by their ability to express enzymatic tools via genetic changes such as mutations, endogenous rearrangements or acquisition of exogenous DNA fragments by plasmids.70 In general, the metabolism of the limited number of synthetic pesticides can be achieved by a single strain, but frequently consortia of microorganisms are necessary for complete degradation.11,71 The biodegradation can be considered as an interesting alternative to physical and chemical methods because it is an eco-friendly and highly efficient approach. Moreover, it may be faster, safer, less expensive and can selectively achieve the complete destruction of organic pollutants.72
Pesticide degradation is dependent on the presence of relevant bacteria, which possess the appropriate enzymatic capabilities. The degradation rates are also strongly influenced by a wide variety of environmental factors including soil type, moisture content, temperature and pH.73 The rhizosphere is the narrow region of soil that is directly influenced by root secretions and associated soil microorganisms. In that particular environment, the activity of these microorganisms is enhanced and may play a significant role in pesticide degradation.74 For example, P-nitrophenol hydrolysis product of parathion was mineralized more rapidly in rhizospheric soils than in non-rhizospheric soils.75 Pesticide degrading microorganisms are endowed with plant growth-promoting activities such as the production of plant growth hormones, biological nitrogen fixation, phosphate solubilization, siderophore and antagonistic properties which playing a key role in the sustainable agriculture.11,76 However, the fast disappearance of pesticide from rhizosphere soils can be attributed to the interaction of different bacteria such as PGPR (plant growth-promoting rhizobacteria) and PGPB (plant growth-promoting bacteria).77 Their ability to degrade pesticide is an important phenomenon through which these chemicals are eliminated from the environment or transformed to less toxic products (metabolites). Furthermore, much attention has recently been paid on bioremediation of contaminated soils with PGPR.78,79 Several studies highlighted the taxonomic diversity of pesticide degrading bacteria including strains of the genera Flavobacterium, Xanthomonas, Enterobacter, Ochrobactrum, Rhododoccus, Agrobacterium, Arthrobacter, Burkholderia, Bacillus, Pseudomonas, etc.66,80 Particularly, members of Pseudomonas genera have been the most extensively studied. Their metabolic versality and diversity make them key players in the depollution of the environment.11,12,15,81 P. putida KT2440 and P. fluorescens, identified as rhizospheric bacteria, were reported as useful for the degradation of several pollutants in the root zone and can even reach deep soil layers.12 Other Pseudomonas species endowed with pesticide degrading abilities and islated from pesticide contaminated agricultural soils are summarized in Table 2.
|
Pseudomonas species |
Origin |
Pesticide |
Rate of degradation (%) |
Medium |
Duration (h) |
References |
|
Pseudomanas sp. |
Rhizospheric |
Quinolphos |
91.2 |
Liquid |
192 |
81 |
|
Pseudomanas. sp. |
Soil |
DDT |
- |
Liquid |
96 |
108 |
|
Pseudomanas sp. |
Soil |
Aroclor1242 |
99.8 |
Liquid |
168 |
6 |
|
Pseudomanas sp. WBG3 |
Soil |
Methyl parathion |
95 |
Soil |
168 |
109 |
|
Pseudomanas sp. |
Agricultural |
Diazinon |
80-92 |
Liquid |
336 |
2 |
|
Pseudomanas sp. |
Agricultural |
HCN (Hexachloro-cyclohexane) |
- |
Liquid |
- |
110 |
|
Pseudomanas putida |
Rhizospheric |
Fenamiphos |
100 |
Liquid |
96 |
111 |
|
100 |
Soil |
192 |
||||
|
Pseudomanas putida |
Agricultural |
Pentachlorophenol |
91 |
Liquid |
168 |
112 |
|
Pseudomanas putida |
coffee cultivated |
Endosulfan |
70 |
Liquid |
480 |
113 |
|
Endosilfan sulfate |
90 |
Liquid |
504 |
|||
|
Pseudomanas putida |
Soil |
Dimethoate |
100 |
Liquid |
96 |
114 |
|
Pseudomanas aeruginosa MCMB‐427 |
Sugarcane rhizosphere |
Dimethoate |
90 |
Liquid |
192 |
115 |
|
Pseudomanas aeruginosa |
Soil |
Chlorpyriphos |
75-87 |
Liquid |
480 |
116 |
|
89 |
Soil |
720 |
||||
|
Pseudomanas aeruginosa |
Soil of cotton |
Endosulfan |
96 |
Liquid |
288 |
117 |
|
Pseudomanas aeruginosa S1 |
Rhizosphere soil |
Glyphosate |
99 |
Soil |
120 |
118 |
|
Pseudomanas fluorescence |
Soil |
Chlorpyrifos |
75-87 |
Liquid |
480 |
116 |
|
89 |
Soil |
720 |
||||
|
Pseudomanas stutzeri S1 |
Soil |
Beta-cyfluthrin |
94 |
Liquid |
192 |
119 |
|
Pseudomanas mendocina |
Black cotton |
Monocrotophos |
73 |
Liquid |
192 |
120 |
|
Pseudomanas brassicacearum |
Agricultural |
Endosulfan |
98 |
Liquid |
360 |
121 |
|
Pseudomanas azotoformans |
Agricultural |
Endosulfan |
82 |
Liquid |
360 |
121 |
|
Pseudomonas pili BG1 |
Soil |
Diazinon |
- |
Liquid |
288 |
122 |
Table 2 Pesticide degrading Pseudomonas origin and degrading ability
Enzymatic degradation of pesticides
The bioremediation process depends on the metabolic potential of microorganisms to detoxify or transform the pollutant molecule using their enzymatic systems. Complete biodegradation of pesticides involves the phenomenon of oxidation by transforming the parent compound into carbon dioxide and water. This process provides both carbon and energy for the microorganism’s growth.68 Each degradation step is catalyzed by specific enzymes involved in the hydrolysis of P-O, P-F, P-S and P-C bonds.5,29,82 The enzymatic activities play an important role because all biochemical transformations are related to the presence of enzymes such as hydrolases, peroxidases, oxygenases, and others.6,12,83 They are indicators of biological equilibrium and changes in the biological status due to soil pollution. These enzymes are produced during different metabolic pathways by degrading cells or are found outside to the cell. Thus the lack of the appropriate enzyme in the soil will stop the process of pesticide degradation which could strengthen the persistance of the pesticide.5 The lack of these enzymes is one of the common reasons for the pesticide persistence. The organophosphate (OP) compound biodegradation has been widely studied and several studies describing OP degrading enzymes are reported29,66 such as cabroxylesterases and phosphodiesterase.84 The enzymatic degradation is the most important strategy for removing pesticides compared to non-enzymatic processes.80 Hydrolases are among the major group of enzymes commonly used in pesticide bioremediation. These enzymes catalyze the hydrolysis of several major biochemical classes of pesticides having esters, peptide bonds, carbon halide bonds, tri-esters, urea, etc. Generally, they are functioning in the absence of redox cofactors making them ideal candidates for current bioremediation strategies.85
The laccase (benzenediol: oxygen oxidoreductase, EC 1.10.3.2) is one of the best-known multicopper enzymes and they have been detected in a variety of organisms such as bacteria, fungi, plants, and insects.86 Mostly, they are extracellular and nonspecific so they have the ability to oxidize a wide variety of aromatic and nonaromatic compounds which are used as hydrogen donors. The laccases have received particular interest in bioremediation applications of treated agriculture soil because they are biodegradable, cost-effective and environmentally friendly. These enzymes are successful at breaking down the complex structures of many pesticides.87–89 The laccase enzymatic activity has been discovered in a small number of bacteria. Some of them were found in P. maltophila, P. syringae, P. fluorescens GB1, P. putida GB1, P. desmolyticum NCIM 2112, P. sp.86 These bacterial laccases have the ability to perform the activity at crucial conditions like in the presence of high salt concentrations and even at alkaline pH values.90 Furthermore, these enzymes can be produced at large scale and can be purified and used for cell-free enzymatic transformation of pesticides.91
Several studies reported the successful and effective degradation of many pesticides catalyzed by the oxidative enzyme laccase in the presence of a reaction mediator (a laccase/mediator system). The results of Maruyama et al.87 showed that over 90% of dymron was degraded in 24h in the presence of ABTS (2.2’-azino-bis [3ethylbenzthiazoline-6-sulphonic acid]), which is used as a mediator rather than by using only laccase enzymes. Pizzul et al.,91 showed that purified laccase from fungi have an effect on glyphosate degradation. They concluded that in the presence of laccase and ABTS, 40.9% of the glyphosate disappeared after 24h, whereas 62.8% of the glyphosate was degraded when Mn2+ and Tween 80 were added together with the enzyme. Besides, Pizzul et al.91 observed a synergistic effect of ABTS, Mn2+ and Tween 80 where 90.1% of glyphosate disappeared after 24h.
Pesticide solubilization by biosurfactants
Once spread into the soil environment, pesticides quickly attach to the mineral and organic matter through a combination of physical, chemical and biological processes. Sorption, complexation, and precipitation constitute the main pesticide-soil interactions. The majority of pesticides are well known for their low aqueous solubility, high hydrophobicity and tendency to stay sorbed in soil.33,92,93 Since water solubility of many organic contaminants is contributing to improving the mechanism removal, surfactants could be added to enhance their solubilization.
Surfactants are amphiphilic molecules structurally varied with two moieties, one hydrophilic and the other hydrophobic. The hydrophilic part generally formed by amino acids, peptides, cations, anions, mono, di or polysaccharides, while the hydrophobic portion is formed of saturated or unsaturated fatty acids. Surfactants are capable of reducing surface and interfacial tensions by accumulating at the interface of immiscible fluids and they can increase the solubility and mobility of hydrophobic or insoluble organic compounds.6 The presence of two functional groups in each molecule is a fundamental physical property of the surfactants, the latter property conditions the formation of micelles in solution by these types of compounds. When the surfactant concentration in water surpasses a certain level called the critical micelle concentration (CMC), surfactant molecule self-aggregate into a cluster known as micelles.94 The formation of micelles in solution gives surfactants their detergents and solubilization properties.
Surfactants, at concentrations above their CMC, have been shown to enhance solubilization of pollutants in soil and have been successfully used in soil washing or soil flushing for remediation of contaminated sites.6,94–96 The overuse of synthetic surfactants in soil remediation could represent an extra source of contamination and could have a serious effect on the ecosystem.97 For this reason, it is mandatory to replace them by biological surfactants generally called biosurfactants (BS) which are biodegradable and nontoxic relative to the synthetic surfactants.98 Additionally, BS could be produced from cheap raw materials and/or the organisms producing them could be genetically modified to overproduce or generate new compounds.98 Based on their chemical configuration, BS are classified as glycolipids, lipopeptides, phospholipids and lipopolysaccharides and are produced by diverse bacterial genera.
To improve soil bioremediation process, several researches were devoted to the isolation and characterization of novel surfactant-producing bacteria. On the other hand, they are focused on the use of BS products, which could solubilize and mobilize pesticide molecules on soil constituents.6,99 The most investigated BS in bioremediation are rhamnolipids, surfactine, lipopeptides and sophorolipids.6
Rhamnolipids are well-studied glycolipids with high potential in agriculture applications.100,101 They have the potential to be a part of alternative strategies to reduce or replace pesticides in agriculture. Nowadays, rhamnolipids plays a great importance for the effectiveness of new biopesticides.101 About 60 rhamnolipid congeners were described.102 Pseudomonas strains are among the best producers of glycolipid containing rhamnose and 3-hydroxy fatty acids.103–105 The most promising bacterial strain, P. aeruginosa, was investigated and advisable as the primary and the best microorganism to produce two classes of rhamnolipids known as monorhamnolipids and dirhamnolipids.104 Other Pseudomonas species have been also reported as rhamnolipid producers such as P. rhizophila, P. teessidea, P. stutzeri, P. putida, P. luteala, P. fluorescens, P. collierea, P. clemencea, P. chlorophis, P. cepacia, P. alcaligenes.76,102
The successful removal of pesticides by rhamnolipids under a variety of environmental conditions compared to synthetic surfactants was well reported.76,105–107 Mata-Sandoval and coworkers105,106 compared the efficiency of rhamnolipid and the synthetic nonionic surfactant Triton-X to remove the pesticides trifluralin, coumaphos, and atrazine from soils with different clay, silt, and sand content. Pesticide solubilization depends on two factors, the first factor is the concentration of the micellar surfactant and the second one is the transportation of organic molecules from the true aqueous phase to a surfactant micellar pseudo-phase. Increased efficacy at concentrations above CMC, but none of the surfactants increased mobilization of pesticides at concentrations below the CMC, due to surfactant adsorption at the soil surface-water interface. The ability of synthetic surfactant to solubilize about twice as much of all pesticides as the rhamnolipid. This is due to the binding more tightly from pesticide to the BS micellar core and its diffusion out to the aqueous phase at a lower speed than that observed for the synthetic surfactant. On the other hand, Wattanaphon et al.107 proved that the use of glycolipid surfactants, produced by Burkholderia cenocepacia, was more efficient for the enhancement of three pesticides solubilization (i.e methyl parathion, ethyl parathion and trifluraline) than commercial surfactants (Tween 80 nonionic surfactant and SDS anionic surfactant).
The use of specific bacteria in the bioremediation process of some specific xenobiotic contaminant is considered as a topical issue in the area of environmental depletion. Pesticides are intensively and massively used in the modern agricultural practices. Pesticides remediation became necessary following their massive use in agricultural soils (with billiards of tons), and the bioremediation processes are preferred to the other physical-chemical ones. Bioremediation approaches are very cheap and with a relatively enduring efficacy. The genus Pseudomonas was taken as a microbial model due to the fact that the various bacteria constituting this genus are metabolically adaptable and able to degrade several and various organic or inorganic substances by producing enzymes and BS that can be used to enhance biodegradation rates.
None.
None.
Authors declare that there is no conflict of interest.

©2020 Hassen, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.