The oxidative degradation of organic matter by H2O2 in the role of Fe2 + under acidic conditions was first discovered by H.J. Fenton in 1894, and then applied to the oxidation of tartaric acid, of which the catalytic system was called the Fenton reagent, and the reaction called the Fenton method [1]. Early research on the Fenton reaction was mainly focused on organic synthesis, enzymatic reactions and the cell damage mechanism [2]. It was noted that in the presence of a catalyst, H2O2 can be efficiently decomposed to generate oxidative active substances with strong oxidizing power, and degrade a variety of organic compounds into CO2, H2O and other small molecules. In 1964, Canadian researcher H.R. Eisenhaner applied it to the degradation of organic matter in wastewater [3], and after that more and more researchers carried out in-depth studies on its use in the degradation of organic pollutants [4-6]. Thus a series type of Fenton reagents was developed, such as light/Fenton reagent [7], ultraviolet-visible light/H2O2/ferric oxalate [8], microwave/Fenton reagent [9], and ultrasound/ Fenton reagent [10]. The mechanism of Fenton reaction and the intermediate species are still under controversial discussion although the Fenton reagent has been researched by many researchers as a powerful oxidant in wastewater treatment, biological systems and natural water. Two different mechanisms, namely radical and non-radical mechanism have been developed from last century. The first and most popular theory, known as the Haber-Weiss mechanism involves the formation of • OH radical in the process of H2O2 reduction [11], from the 1930s, the radical mechanism has been questioned by several studies suggesting that the reaction between H2O2 and Fe(II) produces the ferryl ion species, which is the active intermediate species [12].
Reaction mechanism
The hydroxyl radical mechanism was first proposed by Haber and Willstatter in 1932 [13], which revealed the existence and role of free radicals in the reaction system, and regarded the essence of the reaction as • OH generated by a catalytic during the chain reaction between Fe2+ and H2O2. Throughout the reaction process, the chain initiation phase consists of a series of single electron transfer reactions between Fe2+ and H2O2, • OH and H2O2, • OOH and H2O2 (reaction 1-4), and the generated oxygen free radicals induce the chain growth process (• OH, • OOH). Based on this theory, many scientists conducted extensive research. George [14] detected the presence of O2-• in the study of the KO2--H2O2 system, noted that the dissolved oxygen in the system significantly inhibits the decomposition of H2O2, and proposed that during the study of the mechanism, the role of dissolved oxygen in the Fenton system cannot be ignored [15,16]. Thereafter, Barb [17] and Weiss [18] introduced O2-• into the reaction system, and amended the reaction mechanism, that is, the dissolved oxygen in the system is related to the generation and reduction process of Fe3+, and proposed the reaction (5). Regarding the oxygen generation in reaction (5), Barb & Baxendale [19,20] studied the generation mechanism and kinetic characteristics of oxygen in the Fenton system and finalized the radical mechanism for the Fenton reaction, with the conclusion that the reaction of the system is composed of the following seven elementary reactions (reaction 1,5,6-10) [21].
As understanding deepened on the Fe2+/Fe3+ hydrolyzate and ion forms (Fe(H2O)62+/Fe(H2O)5(OH)+/Fe(H2O)4(OH)2/FeOH2+/Fe(OH)2+/Fe(OH)24+), and with the wide use of dynamics, marking and spectroscopy [22-24], extensive research on the ion forms of Fe2+/Fe3+ in the system were also carried out, and it was concluded that the dissociation and electron transfer of the association product are simultaneous processes, and produce a variety of free radicals with strong oxidization. Based on the current understanding of the complication process of high concentration H2O2-H2O systems, Jones et al. [25] introduced the product of Fe2+/Fe3+/hydrolysis/complex/association into the Fenton elementary reaction, and believed that, the association degree of H2O2 and Fe2+/Fe3+ is determined by the concentration of H2O2, which means that at low concentrations, the three substances can generate associatively [Fe2+/3+(H2O)5(H2O2)] in the aqueous solution, and at high concentrations, they can generate bimolecular and even multi molecular association products [Fe2+/3+(H2O)5(H2O2)n](n≥2). At present, the more consistent view on the understanding of Fe3+ and H2O2 is that the associated Fe3+ can further associate with H2O2 through the transfer of its inner electrons, to generate a compound with single-ended linear or ring configuration (reaction 11-12).
Study of the system shows that:
- Fe3+ can significantly affect the dissociation rate of H2O2 with the action of its dissociative products, which may be with iron-oxide intermediate Fe3+HO2- or FeO3+ [26-29];
- The decomposition rate of H2O2 to produce O2 is related to the types of complexes [30-32];
- The intermediates in H2O2 decomposition include Fe3+(H2O)5O2H- and Fe3+(H2O)4(H2O2)O2H-, with the former product as the main type in high concentration [33].
There still remain controversies on the understanding of the association system of Fe2+ and H2O2, and currently the following evidence can confirm indirectly that the association process of Fe2+ and H2O2 is achieved by the transfer of inner electrons:
- Fe2+ must provide more than one complex site in complex with a macrocyclic ligand, and in such conditions thermodynamically unstable H2O2 will be produced when outer electrons are transferred, so from the perspective of thermodynamics, the mechanism of inner electron transfer sounds more feasible [34-36];
- The modeling analysis on kinetics shows it reasonable to generate the product of Fe2+- H2O2 by inner electron transfer at pH> 4 [37,38];
- It can be concluded that Fe (H2O2)2+ is more stable than Fe (H2O2)O+, Fe(OH)2+, and Fe(H2O)2+ in the gas phase by analysis of charge stripping mass spectrometry combined with the as initio method [39].
Kinetics
The Fenton system may cover more complex reactions, and now researchers focus on its kinetics study in order to understand properly the role of various factors in it, further clarify its reaction mechanism, evaluate the impact of water quality factors on the reaction process, and provide valuable references for its practical application. Gallard et al. [40] established an equation of Fe (II) reaction rate (equation 13), and systematically studied the effect of pH on the system, hypothesizing that pH mainly affects the reaction rate of Fe(II) with H2O2 and the ionized form of HO2·/O2·-, and thus established the following three equations (equations 14-16) to simulate the degradation process of organic compounds via this system. The results showed that at low pH (pH <3), the simulation predictions coincided with the experimental results, but as the pH value increased, the predicted concentration of organic compounds was higher than the experimental value, therefore the authors speculated that there might be other possible reaction pathways and mechanisms in the conditions of high pH. Based on the good consistency of this model with the actual situation at pH less than 3, Gallard et al. [41] further simulated the kinetics of Fe3+ and H2O2, and concluded that during the reaction of Fe (III) with H2O2, they formed a complex first and then this complex decomposed to Fe2+ and HO2• (reaction 17-20), and accordingly proposed a more elaborate kinetic model (equations 21-25). By using the model, they simulated the catalytic decomposition reaction of Fe (III) on H2O2 at pH less than 3. The results showed that the pH value and the concentration ratio of Fe (III) to H2O2 can affect the decomposition process of H2O2 significantly, as the pH increases, the decomposition rate of H2O2 increases accordingly. When 1≤CH2O2/C Fe(Ⅲ)≤50, the decomposition rate of H2O2 is proportional to it; when
, the decomposition rate of H2O2 is unaffected substantially; when
the decomposition rate of H2O2 is inversely proportional to it, and the actual degradation process of waste water proved that the model basically agrees with the experimental results [42].
Other relevant reports were also published on the impact of other ions in the system on its reaction kinetics. Based on the elementary reactions of the classical Fenton system, the De Laat research group [43] established a decomposition kinetics model of H2O2 in the presence of SO42- by the complexation reaction of SO42- and Fe3+, and concluded that at higher concentrations of SO42-, SO42- and Fe3+ formed a complex with very low activity and hindered the complication of Fe3+ and H2O2, resulting in a decline in efficiency of the Fenton system. Walling et al. [44] studied the effect of Cu2+ on the oxidation of organic compounds in the Fenton system. The results showed that the presence of Cu2+ will change the next reaction pathway of organic free radicals generated. When the system is free of Cu2+, there are three possible reaction pathways for the oxidization of organic compounds into organic radicals (R •), the first is that Fe2+ is oxidized by R • into Fe3+, the second is that Fe3+ is reduced into Fe2+, the third is that there are mutual polymerizations between radicals. When Cu2+ is introduced into the system, it can react with R • and reduce its concentration in the system, thereby changing the reaction progress.
To meet the needs of practical applications, the studies on kinetic models of this system were reported increasingly for degradation of specific organic pollutants. Chen et al. [45] studied the phenol degradation process via this system and presented the corresponding degradation path, with improvement on the degradation mechanism of aromatic compounds and the practicability of the Fenton system. The author believed that the phenol is first attacked by • OH to generate the radical of dihydroxy cyclohexadiene (DHCD•), after that DHCD• will be dehydrogenized into hydroquinone (HQ). HQ with strong reduction ability can react quickly with Fe3+ to produce semiquinone radical (SQ •), and SQ • will continue to reduce Fe3+ into benzoquinone (BQ), at last BQ will be reduced into SQ • by DHCD •. In the above process, the quinone is an intermediate which will accelerate the circulation of Fe3+/Fe2+ and promote the generation of Fe2+. The whole process is shown as an autocatalytic process (Figure 1). The Duesterberg research group [46] investigated the autocatalytic process of the oxidation on the hydroxy acid by the Fenton system. Based on the Pignatello research model, they revised and added some reactions, adjusted the rate constants of radical reactions, and established a kinetic model of the oxidation on the hydroxy acid by the Fenton system. The results also proved the role of quinone intermediates in the catalytic degradation process of aromatic compounds (Figure 1).
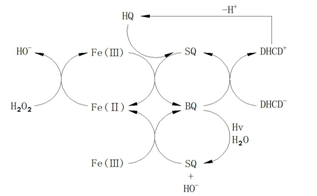
Figure 1: Quinone intermediates electron transfer in the process of phenol oxidation.
- Reaction mechanism of ferric Ion
The mechanism of ferric ion was first proposed by Bary & Gorin [12] in 1932, in which they believed the reaction pathway to generate Fe (IV) might be in the Fenton system and the relevant process were as follows (reaction 26,27):
After that, more and more experiments proved that under certain conditions the Fenton system can produce ferric ions with oxidization properties. In the 1940s, George [47] and Abel [48] questioned the hydroxyl free radical mechanism successively and argued that this system may exist other oxidizing substances, such as ferric ions. Kremer [49] questioned the form of ferric ions in solution in the hydroxyl free radical mechanism. According to this mechanism, the iron is in the form of ferrous and ferric ions in the system, and it was speculated that at low concentrations of hydrogen peroxide, the reaction (28) is less likely to occur, and most of the hydroxyl radicals generated in the reaction (29) will react with ferrous ions to produce ferric ions, and then the ferric ions will continue to react with hydroxyl radicals according to the reaction (30). If the reaction does occur in the system, it proves that Fe3+ and hydroxyl radicals cannot exist alone in the system, both will be further reacted into FeOH3+, the protonated form of FeO2+ (FeO2++H+—FeO2+), which also suggests the possible presence of ferric ions from the other side.
(28)
(29)
(30)
In the last two decades, studies have focused a lot of attention on the oxidation of organic pollutants by the Fenton reagent under neutral conditions, and regarding the reactive mechanism under neutral conditions, many researchers have also carried out extensive investigation of the generation and reaction mechanism of ferric ions. During the study on the oxidation process of H2O2 on cytochrome C catalyzed by the complex of Fe (II) and EDTA, Koppenol et al. [50] found that the reaction characteristics of this process are completely different from those of the oxidation process hydroxyl radicals produced by pulse radiolysis on cytochrome C, and they also concluded that the intermediate with oxidation activity may be present as ferric ions. O Pestovsky et al. [51] excluded the possibility of tetravalent iron existence under the conditions of pH≤3 by use of different principles on the respective reactions of (CH3) 2SO to tetravalent iron and hydroxyl radicals. But under the conditions of pH greater than 3, more and more evidence has proved the existence of tetravalent iron in the system. Stephan et al. [52] studied the oxidation process of As3+ in natural water by the Fenton reagent, and found that under conditions of pH=3.5-7.5, the hydroxyl radical scavenger propanol and formate, do not have a significant impact on the oxidation reaction, so they believe that there are other reactive oxidizing substances in this system and presume it is ferric ion. Laurent et al. [53] simulated the conversion conditions of Fe2+ and Fe3+ ions during the Fenton reaction within the droplets in clouds under two different mechanism conditions, and found that both mechanisms can explain well the process of iron conversion. They also believe that during the reaction the concentration of ferric ion is higher than that of hydroxyl radicals in four orders of magnitude, so that the ferric ion dominates in the reaction. Rahhal et al. [54] found some phenomena against the nature of hydroxyl radicals in the catalytic decomposition process of H2O2 by the complex of Fe (II) and diethyl pentaacetic acetic acid that is the active oxidative intermediate produced in the experiment can be inhibited by t-butanol, so they presumed that the active oxidative intermediate in the system is ferric ion. During the study on the role of NO in oxidation of organic compounds by the Fenton reagent, Sharpe et al. [55] noted that in the reaction, Fe2+ and hydrogen peroxide will first produce two different ferric ions: FeO2+ and FeOH3+, then they will hydrolyze or directly break bonds into hydroxyl radicals, but also pointed out that in the reaction no product generated by hydroxyl radicals and tracer was observed. Walling et al. [56] believe that in this system metal ions will first form a complex with H2O2, the nature of which will decide the following reaction mechanisms: (1) if single electron transfer occurs in the complex of Fe and H2O2, Fe will lose an electron, and produce hydroxyl radicals and the reaction will proceed as the hydroxyl free radical mechanism; (2) if double electron transfer occurs in the complex generated, Fe will lose two electrons, and form strong oxidizing ions with higher valence, then the reaction will proceed as ferric ion mechanism; (3) if the generated complex itself has strong oxidation, the complex in the whole system is the main active oxide intermediate, but this theory has not yet been confirmed experimentally and theoretically.
Currently, in the actual study, since hydroxyl radicals are easily observed in the electron spin resonance (ESR) spectrum, and the reaction kinetics may also be described by the oxidation of hydroxyl radicals, most researchers prefer the hydroxyl free radical mechanism, but in the presence of complexing agents or in some neutral systems, the hydroxyl free radical mechanism alone does not adequately explain some of the special phenomena in the experiment. In the above conditions, more and more researchers began to favor the ferric ion mechanism. Actually, the Fenton system is used widely in chemical, biological and environmental systems, and it is unlikely that this is with the same mechanism all the time. Sometimes two mechanisms may co-exist in different systems, or some kind of mechanism may be dominant, depending on the specific nature of the system. Further research on the reaction mechanism of the system should focus on direct evidence for the existence of an active intermediate by use of the latest online analysis and detection combined with tracking tools and techniques. On this basis, the next work should focus on the catalytic activity and the degradation efficiency of H2O2, to research the impact of other assistive technology (photocatalyst/ultrasonic/ultraviolet, etc.) on the active intermediate in the system, and to provide theoretical guidance for the efficient industrial application of this technology.


