MOJ
eISSN: 2573-2919


Short Communication Volume 10 Issue 1
Universidad Tecnológica de Izúcar de Matamoros, Mexico
Correspondence: Amado Enrique Navarro-Frómeta, Universidad Tecnológica de Izúcar de Matamoros. Calle Reforma 168, Barrio de Santiago Mihuacán, C.P. 74420, Izúcar de Matamoros, Puebla, Mexico
Received: February 01, 2025 | Published: February 12, 2025
Citation: Crespo-Barrera PM, argas-Santos A V, Navarro-Frómeta AE. Optimization of a column chromatography separation method for spectroscopic analysis of polycyclic aromatic hydrocarbons present in air particulate matter. MOJ Eco Environ Sci. 2025;10(1):21-23. DOI: 10.15406/mojes.2025.10.00342
Air pollution is one of the greatest concerns worldwide, as it affects world population evenly and it is the cause of death of children younger than 5 years. The presence of organic pollutants in particulate matter is well known, and as its composition is studied, new methods are developed, and others are optimized to reduce the analysis time. In this sense, this communication aims to describe the optimization of a column chromatography separation method for the easing of the UV-Vis analysis of the organic pollutants present in total suspended matter in air samples taken in two different Mexican cities.
Keywords: column chromatography, UV-Vis, air pollution, organic microcontaminants in air
According to the World Health Organization (WHO) around 99 % of the world population breath air with some degree of pollution.1 Recent studies show that ozone, particulate matter and nitrogen oxides exposure are increasing at a global level,2 affecting vulnerable population and increasing the respiratory3 and neurological4 diseases associated with the exposure.
Column separation is used for chemical analytic essays, as it offers a wide range of options of separation mechanisms depending on the particle size and mechanism for separation.5 This method had been used for separation of polycyclic aromatic hydrocarbons (PAHs) in aerosols a few years ago6,7 and it is very useful because pollutants present in air are abundant and of different nature, as they include both anthropogenic and natural substances, which make difficult its analysis and study. In particular, PAHs are a variety of organic chemicals with different number of carbon rings which determines their bioaccumulation and persistence in environmental matrixes.8 Moreover, PAHs are associated with particular matter,9 so they can be detected when air samples are taken.
There are a variety of methods for PAHs quantification10–13 many of which require standards acquisition or high cost equipment. However, there also exist methods for quantification of PAHs based on UV-Vis signals, which are in good agreement with the results found by adsorption chromatography (differences lower that 3%).14 In this sense, this work aims to optimize a column separation method to easing the study of PAHs and other pollutants commonly found in air samples, making the quantification by UV-Vis.
Reagents and equipment
Deuterated standards of chrysene (d10-Ch) and acenaphthene (d10-Ac) were purchased from Sigma Aldrich. Water, hexane, diethyl ether (DE), acetone, dichloromethane (DCM) and methanol (MeOH) were purchased from J. T. Baker. All reagents were HPLC grade and used as received.
The air samples were taken with an air sampler TE-1000-PUF from TISCH Environmental. The polyurethane foam (PUF) and 10 mm quartz filters were also purchased from TISCH Environmental. The equipment, PUF and filters were cleaned according to the TO-13A EPA method.15 Briefly, the filter was treated at 450 °C for 5 h and the PUF was cleaned for 2 h with acetone and for 16 h with hexane:DE 10:1 v/v (DEH) in a Soxhlet apparatus. All the crystalware used and components of the air sampler were also cleaned following this method.
Sampling
The evaluation of the method was optimized using two air samples from different locations in Mexico, in order to guarantee the separation of the different pollutants independently of the concentration and interaction with other substances present on them. The results presented in this work correspond to a 24-h sample taken in Puebla, Mexico (PUE) and an 8-h sample taken in Xalapa, Mexico (XAL). PUE sample is located in a semi-rural area whereas XAL corresponds to an urban area.
Sample extraction and column separation
Before extraction, the PUF were injected with the deuterated standards, to account for the recovery of the samples. Then, the PUF and filter were extracted together for 18 h with the DEH mixture in a Soxhlet apparatus. After this time, the samples were quantitatively transferred to a pear flask to rotoevaporate the sample until reaching a 0.5 mL volume. After extraction, the samples were placed in the alumina-activated column. They were eluted with 7 mL of a mixture hexane: DCM 9:1 v/v (HDCM9), then with 10 mL of a mixture hexane: DCM 1:2 v/v (HDCM1) and, finally, with 7 mL of MeOH. For the PUE sample, the elutions were collected together but for XAL, each milliliter of each elution was collected separately and measured individually in the UV-Vis spectrometer to evaluate the separation.
Concentration calculations
The UV-Vis measurements were made in a Genesys 50 from Thermo Scientific with a swap from 200 nm to 1100 nm, at 2 nm resolution. The concentration of the PAHs was determined by solving an equation system explained by Navarro Frómeta et al.,16 separating monocyclic (Mon), naphthalenes (Nap), phenanthrenes (Phe), anthracenes (Ant), pyrenes (Pyr), and chrysenes (Cry) according to the extinction coefficient of each PAH, which values are presented in Table 1.
|
λ (nm) |
Mon |
Nap |
Phe |
Ant |
Pyr |
Cry |
|
200 |
40 000 |
14 500 |
17 000 |
8 000 |
48 000 |
|
|
218 |
10 100 |
45 000 |
26 200 |
14 900 |
12 250 |
27 000 |
|
230 |
2 000 |
110 000 |
8 700 |
8 100 |
64 000 |
13 800 |
|
240 |
80 |
2 800 |
34 000 |
30 000 |
123 500 |
15 840 |
|
255 |
258 |
2 400 |
55 600 |
100 000 |
18 700 |
55 000 |
|
275 |
250 |
4 900 |
13 200 |
2 900 |
76 500 |
70 000 |
|
285 |
0 |
4 900 |
8 200 |
1 500 |
6 100 |
12 100 |
|
295 |
0 |
3 349 |
12 300 |
412 |
8 164 |
12 000 |
|
338 |
0 |
0 |
200 |
2400 |
77 550 |
500 |
|
375 |
0 |
0 |
0 |
7 240 |
100 |
100 |
Table 1 Molar extinction coefficient, in L/mol*cm, for the different groups of PAHs
The extinction values are used to create a matrix where the PAHs concentrations are the unknowns. The concentration of each PAH was calculated using the absorbance values obtained from de UV-Vis analysis for each specific wavelength: 218 (Mon), 230 (Nap), 255 (Phe), 272 (Ant), 338 (Pyr) and 375 nm (Cry). The extinction values matrix is inverted to generate the equation system, and the concentration is obtained with the inverted value times its corresponding absorption value. These values are multiplied by the carbon number of each PAH and then multiplied by the molecular mass of Carbon.
The PUE sample separation results are plotted in Figure 1. It is clear that when the elutions are collected together, no clear separation is obtained. All PAHs are present in all fractions, with higher values in HDCM1, except for Mon which is higher in MeOH and Phe, which is higher in HDCM9.
On the contrary, when each milliliter of each elution is collected separately (Figure 2), the cut-off points are clearer. It is evident that Mon are strongly separated by HDCM9 (Figure 2A), together with the first two mL of HDCM1, Phe and Pyr by the 3rd to 10th mL of HDCM1 (Figure 2B) and Nap and Ant by MeOH (Figure 2C). However, due to the nature of the PAHs there is still a mixture of almost all PAHs on each fraction, but difference in magnitude orders among concentration of each compound lead to clear cut-off points which could be used to enhance further analysis of these types of samples.
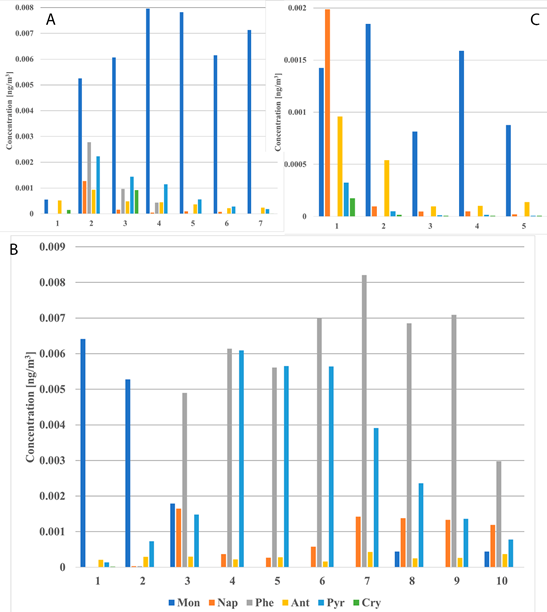
Figure 2 Comparison of concentration of PAH calculated for each milliliter collected separately from XAL sample.
For Cry, it can be seen that its concentration is higher in HDCM9 and it can also be found in the first mL of MeOH, but it is not found in the 3rd to 10th mL of HDCM1. A similar trend is followed by Phe, which is only present in 2nd, 3rd and 4th mL of HDCM9 and then again from 2nd to 10th mL of HDCM1. The remaining PAHs are present in almost all fractions but varying their concentration all along.
It is worth mentioning that other organic micropollutants other than PAHs may be found in samples of atmospheric particulate matter.16 These compounds may contribute to absorption at the wavelengths used and to inaccuracies in the concentrations of some types of PAHs, given that the UV-VIS spectrophotometry method was developed with mixtures of hydrocarbons. A more detailed study of this, using gas chromatography coupled with mass spectrometry, would allow to assess the degree to which the determination of the different types of PAHs is affected. This study is currently under development. However, what is described in this work can be used in laboratories that do not have more complex equipment for the analysis of the hydrocarbons present in atmospheric particulate matter.
Finally, it is worth noting that the difference between the total PAHs concentration is 3 magnitude orders in XAL, compared to the PUE sample. This exhibits the urban and semi-rural characteristics, respectively, of each analyzed sample.
Column chromatography is a powerful method to separate PAHs mixtures, as those found in air samples. The optimization process requires collecting all HDCM9 together with the two first milliliters of HDCM1 for Mon analysis. The remaining 7 mL of HDCM1 separates Phe and Pyr. Finally, Nap and Ant were separated in MeOH. However, Nap is present in HDCM1 and should by also quantified in this fraction.
PMCB thanks to CONAHCyT for the postdoctoral scholarship 710042. And PMCB and AENF thank to Dra. Elizabeth Hernández for the access to the XAL sample.
None.
The authors declare no conflict of interest in writing the manuscript.

©2025 Crespo-Barrera, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.