MOJ
eISSN: 2573-2919


Research Article Volume 5 Issue 6
1Department of Plant Pathology, Agriculture and Forestry University, Nepal
2Department of Entomology, Institute of Agriculture and Animal Science, Nepal
Correspondence: GC Sagar, Department of Plant Pathology, Agriculture and Forestry University, Nepal
Received: September 15, 2020 | Published: November 19, 2020
Citation: Sagar GC, Manandahar HK, Shrestha S, et al. In-vitro and greenhouse management of banded leaf and sheath blight (BLSB) of maize, at Rampur, Chitwan, Nepal. MOJ Eco Environ Sci. 2020;5(6):238-242. DOI: 10.15406/mojes.2020.05.00199
The research included in-vitro experiments on the effect of the fungal antagonist (Trichoderma spp.) and fungicide (carbendazim) against BLSB pathogen (Rhizoctonia solani); and greenhouse experiment for the management of BLSB using antagonists (Trichoderma and Pseudomonas) and a fungicide (carbendazim) in different combinations. The in-vitro and greenhouse experiments were done at the plant pathology laboratory and greenhouse of Agriculture and Forestry University, Rampur, Chitwan.
Of the isolates of Trichoderma evaluated against the BLSB pathogen in dual culture, the Trichoderma isolated from between healthy and diseased maize plants gave the maximum growth inhibition percent of the pathogen at 24 (19.86±0.52), 48 (51.63±0.40) and 72hours (72.78±1.11). Carbendazim at 10 ppm completely inhibited the growth of both the pathogen and Trichoderma on PDA medium, while at 1 ppm, the growth inhibition of the pathogen was (54.22±0.89) percent and of Trichoderma was (50.22±3.11) percent.
The management experiment in a glasshouse with seven treatments was done in a randomized complete block design with three replications. Carbendazim alone gave the highest disease control (59.73%) with the lowest disease severity (28.67±0.67) and AUDPC per day (803.33±33.33). A combination of treatments alternately with Trichoderma and carbendazim also had a significant effect on reducing disease severity. In a separate experiment, Trichoderma with and without pathogen gave significantly higher shoot length, root length, shoot weight and root weight when compared with untreated control.
Keywords: antagonist, in-vitro, inhibition, severity, disease control, Trichoderma
Maize (Zea mays L.) belongs to the order Poales and family Poaceae. In Nepal maize in terms of production and area is the second most important crop grown for food, feed, and fodder and is the main cereal crop grown in hills and mountains.1,2 Of the different diseases of maize reported, the disease banded leaf and sheath blight (BLSB) caused by Rhizoctonia solani (teleomorph: Corticium sasakii, syn. Thanatephorus cucumeris) was first reported by Bertus in 1927 from Sri Lanka under the name sclerotial disease.3 Under unfavorable conditions, BLSB reached up to 40.5 percent loss in grain yield.4 With disease severity level up to 87.3 percent, there was grain yield reduction up to 31.9 percent in maize cultivars.5 Pathogen is capable of infecting the plant in all the growth stages from seedling stage of crop growth to maturity of the crop. Symptoms starts with the appearance of large and areas discolored with alternate and irregular dark bands. At first disease are developed on the first and second leaf sheath above the ground which after development spreads to ear causing ear rot. Disease on maize plants appears at its pre-flowering stage (30-40 days old plant) but the disease can be seen also on younger plants. The disease after its occurrence causes direct loss due to premature death, stalk breakage, destruction of leaves, leaf sheaths and ear rot.6 The disease symptoms are seen on all the aerial parts of the maize plant other than the tassel.
There are several methods viz., physical, cultural, chemical, and biological, by which the disease BLSB can be managed. Removal of a leaf at the base, use of bio-control agent (Pseudomonas, Gliocladium, Trichoderma, Penicillium, Aspergillus), use of different chemicals and botanicals can manage this disease.
Biological control has become a necessary component for the safe and effective disease management of the plant. Trichoderma harzianum effectively suppressed both, sclerotial formation and growth of R. solani.7 There was 80 percent inhibition in mycelial growth after 72 hours of incubation and 35.5 percent inhibition information of sclerotia after ten days of incubation of Rhizoctonia solani by T. harzianum.8 The species of Trichoderma can produce extracellular enzymes or antifungal antibiotics, or they may also be competitors to fungal pathogens, promote plant growth, and induce resistance plants.9 These may also play a major role in mycoparasitism because of changes in cell wall integrity. Trichoderma spp. produces a large variety of volatile secondary metabolites such as ethylene, hydrogen cyanide, aldehydes and ketones which play an important role in controlling the plant pathogens.10 Antimicrobial activity of the Pseudomonas fluorescens had been reported against numbers of fungi.11 Vidhyasekaran et al.12 reported the powder formulation of the microbes P. fluorescens effectively controlled the rice blast disease both infield and glasshouse conditions. P. fluorescens controlled P. oryzae and R. solani by agar plate method. Ahuja & Payak13 reported the effectiveness of carbendazim, validamycin A, aureofungin, dichlroline, benodamil, thiophanate methyl and thiobendazole on the isolates of R. solani. The most effective control of the sclerotial stage of the disease was with Bavistin 50 WP (carbendazim) giving 87 percent of the disease control which was followed by Brestan 60 WP (fentin), Calixin 75 EC (tridemorph), Difolatan 80 WP (captafol) and Benlate 50 WP (benomyl) with 77, 74, 72 and 32 percent disease control, respectively.14
Laboratory experiments
Laboratory experiments were carried out at the laboratory of the Department of Plant Pathology, Agriculture and Forestry University, Rampur,Chitwan. In the Laboratory experiment 1 (Dual culture of R. solani and Trichoderma), Trichoderma used for dual culture with R.solani were Trichoderma isolate Salyan (source: Plant Pathology Department, AFU), Trichoderma isolated from root zone between healthy and diseased maize plant, Trichoderma isolated from the root zone of the diseased maize plant and Trichoderma isolated from the root zone of the healthy maize plant.
In Laboratory experiment 2 (Effect of different doses of carbendazim on the growth of R. solani and Trichoderma), The best performing biocontrol agent based on dual culture studies in experiment 1 and the pathogen was tested to find its compatibility against used fungicides at different doses by following poisoned food technique in PDA medium as given by Nene and Thapaliyal15 and percent inhibition of mycelial growth was calculated. For R. solani 1, 10, 50 and 100 ppm of carbendazim and for Trichoderma 0.5, 1, 10 and 50ppm of carbedazim were used.
Glasshouse experiment
Evaluation of carbendazim and biocontrol agents for disease control was laid on randomized complete block design (RCBD) by sowing seeds of maize variety Arun 2 in pots. Two seeds were sown in each pot and the only single plant was maintained one week after germination. There were five pots in one treatment and each plant was taken as a sample. There were seven treatments with three replications. The diameter of a pot was 30 cm. The disease scoring was done according to the scale given.2,16
Disease rating scale
The area under disease progress curve
Area under disease progress curve (AUDPC) was calculated by using the following formula.17
Where Yi= disease severity on the ith date
Yi+1=disease severity on the i+1th date and
n=number of dates
Preparation of inoculum of Rhizoctonia solani
The test pathogen was purified and multiplied on sorghum grains. Sorghum grains were pre-soaked in 2 percent sucrose solution overnight, drained and boiled in freshwater for 30 minutes, and drained again. This was transferred into 1000ml flasks at the rate of 400g and autoclaved at 15lb psi (12˚C) for 20 min. The flasks were allowed to cool at room temperature and inoculated with 5mm discs of 3 to 4-day old culture of R. solani grown on PDA. Seven discs per flask were added and the flasks were incubated for three weeks at 28±2˚C. Four grains were placed in between leaf sheath and stalk of 40-day old maize plants maintaining high humidity during disease development by frequent watering and flooring with water-soaked jute sacks. Five plants of each treatment of each replication were tagged for disease observation and coring of disease, calculation of disease severity and AUDPC were done.
Glasshouse experiment 2 - Evaluation of carbendazim and biocontrol agents on growth promotion of maize seedlings
Maize seeds were treated with carbendazim and biocontrol agents and were sown in a pot as described in a glasshouse experiment. The experiment was laid in a completely randomized design(CRD).
For the chemical seed treatment, 100g of seeds were dipped in a 100ml of water mixed with 1g Bavistin (carbendazim) powder and was left for the whole night. For the seed treatment with Trichoderma, 5ml of sterilized distilled water was poured in 4-day old Petri plates with Trichoderma. Trichoderma from the petriplate was scrapped and mixed with 95ml of water. Then 100g of maize seeds were dipped in the suspension for the whole night. In the case of Pseudomonas 0.5ml of commercial formulations of Pseudomonas fluorescens (Bio Cure-B) was spread with driglaski spatula and incubated for two days. The bacterial growth was scrapped with the help of a spatula and mixed with 100ml water and 100g of maize seeds was soaked in the bacterial suspension for the whole night. For treatments with R. solani, soils were inoculated with four sclerotia per pot on the same day of seed sowing. Ten days after germination, root length and shoot length of the seedlings were measured. Seedlings were oven-dried for measuring root weight and shoot weight.
Statistical data analysis
The agronomical, disease scoring, seed treatment, and in-vitro test data were tabulated in an excel datasheet. All the recorded data were subjected to analysis by using the reference of Gomez & Gomez (1984). The data were processed to fit into R-studio and analyses were conducted using R 3.0.318 and the agricolae version 1.1-8 package.19 The data entry was done to develop the ANOVA table and different treatments were compared by Duncan’s multiple range test. All the figures and graphs were prepared by using Microsoft excel 2013.
Laboratory experiments
Dual culture of R. solani and Trichoderma: Trichoderma isolates isolated from inbetween the diseased and healthy maize plant gave the maximum inhibition percent of R. solani at 24 (19.86±0.52), 48 (51.63±0.40) and 72 (72.78±1.11) hours followed by the isolate from healthy maize plant and the isolate from Salyan (Table 1). The isolate from diseased maize plant gave the least inhibition percent at 24 (10.90±0.70), 48 (36.86±0.53) and 72 (60.55±0.27) hours. The result was in agreement with Rajput (2016) who stated that Trichoderma harzianum caused 80 percent inhibition of mycelial growth after 72h of incubation; it also caused 35.5 percent inhibition of sclerotial formation after 10 days of incubation. These results are in agreement with Kumari et al. (2016), who reported more than 50 percent inhibition of mycelial growth of R. solani by dual culture test.
Effect of different doses of carbendazim on the growth of R. solani and Trichoderma
At 1 ppm concentration of carbendazim the growth of both the R. solani and Trichoderma was nearly half of the control i.e. without carbendazim (Table 2) (Table 3). At 10 ppm and above the growth of both the pathogen and antagonist was completely inhibited. The results of the present work are in agreement with the findings of (Vyas, 1994)20 who reported the compatibility of Trichoderma viride with carbendazim. According to Gowdar et al.,20 there were only 65.63 and 28.56 percent inhibition in the growth of Trichoderma when placed in a media with 0.1 percent carbendazim.
When biocontrol agents and chemical control methods are integrated they bear the potentiality of controlling the plant pathogens with minimal interference with the biological equilibrium and success of biocontrol method is possible only if biocontrol agents are compatible with the herbicides and the fungicides.21
Glasshouse experiments
Effect of carbendazim and biocontrol agents for disease control
Effect on disease severity (%): There was no significant difference between treatments against banded leaf and sheath blight (BLSB) disease severity on 60 DAS whereas 70 DAS, 80 DAS, and 90 DAS showed a significant difference (Table 4). On 70 DAS carbendazim showed the lowest disease severity (26.67±2.00) which was at par with Trichoderma (32.67±2.667), Pseudomonas (32.67±3.33), Trichoderma-Carbendazim-Trichoderma (28.67±2.00) and Pseudomonas-Carbendazim-Pseudomonas (32.67±0.667). The highest disease (41.33±2.00) was observed in untreated control which was at par with Pseudomonas-Carbendazim-Trichoderma (35.33±4.667).
On 80 DAS, carbendazim showed the lowest disease severity (32.00±2.667). The second-lowest disease was with Trichoderma-carbendazim-Trichoderma (40.00±3.33) which was at par with Trichoderma (46.00±4.67), Pseudomonas (32.67±3.33) and Pseudomonas-carbendazim-Pseudomonas (32.67±0.667). The highest disease severity was found in the untreated control (48.67±4.00) which was at par with Pseudomonas-carbendazim-Pseudomonas (47.33±0.667).
On 90 DAS, carbendazim showed the lowest disease severity (28.67±0.67). The second lowest disease was with Trichoderma-carbendazim-Trichoderma (44.67±3.33) which was at par with Pseudomonas (48.67±4.67). The highest disease severity was found in untreated control pots (71.33±2.667). The third lowest disease was with Pseudomonas-carbendazim-Pseudomonas (57.33±2.00) which was at par with Pseudomonas-carbendazim-Trichoderma (58.00±4.67) and Trichoderma (60.67±5.330).
The banded leaf and sheath blight were first noticed at the 60 DAS. A gradual increase in the disease severity (%) was continued up to 70 DAS. After that, the disease severity increased at a decreasing rate on 80 DAS and 90 DAS on all the treatments except carbendazim. In carbendazim disease severity decreased from 80 DAS to 90 DAS. Disease severity was highest on 70 DAS, 80 DAS and 90 DAS in the untreated control (Figure 1).
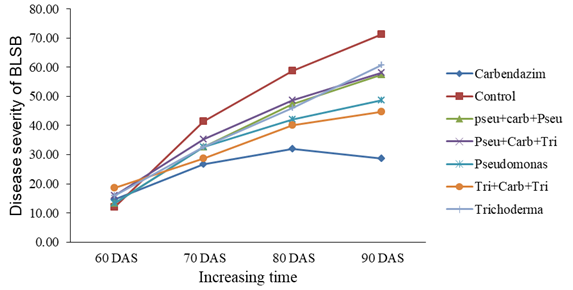
Figure 1 Severity (%) of banded leaf and sheath blight (BLSB) disease of maize to time for different treatments.
Disease control percent by various treatment to untreated control
Carbendazim gave the highest disease control on 70 DAS (35.44%), 80 DAS (45.58%), and 90 DAS (59.73%) compared with untreated control (Table 5). The second highest control was obtained with Trichoderma+carbendazim+Trichoderma when applied alternately at 10 days interval. Sinha14 and Shivani (2017) also reported carbendazim (Bavistin) as the most effective fungicide for the control of BLSB disease in maize.
The above results showed that carbendazim was found to be the most effective in controlling the disease in comparison to other treatments. Compared with untreated control the second-best control was obtained with Pseudomonas and Trichoderma+carbendazim+Trichoderma. However, all treatments reduced the disease severity when compared with untreated control on both the 80 and 90 DAS.
Akthar et al.22 reported the least disease severity percentage (25.78%) with highest grain yield (31.50 quintal/ha) when carbendazim was used as a foliar spray with 0.1 percent concentration. Others reported that several chemicals like carbendazim, validamycin, Topsin M, benodanil, Rhizolex etc inhibit the growth of the mycelium and reduce disease severity percentage in both in-vitro and field conditions.13,23
Karkee and Mandal24 also reported the control of mycelial growth of pathogen Rhizoctonia at lower concentration in their study.
Effect on AUDPC
There was no significant difference among treatments against banded leaf and sheath blight (BLSB) for AUDPC1but significant for AUDPC2 and AUDPC3 (Table 6). The lowest total AUDPC was found in carbendazim (803.33±33.33) which was significantly different from all other treatments. Remaining other treatments was significantly at par but different from untreated control.
Effect of treatments on AUDPC per day
BLSB disease progress per day was highest in untreated control followed by Trichoderma, P+C+T, and P+C+P. Disease progress per day was least in carbendazim treated pots,T+C+T and Pseudomonas (Figure 2).
Effect of carbendazim and biocontrol agents on growth promotion of maize seedlings
The effects of biocontrol agents on the growth promotion of maize seedling were significant under both with and without pathogen (Table 7). However, the effects do not seem to be different between with and without pathogen though the trend is a bit higher with the antagonist alone (without pathogen). Trichoderma gave the longest shoot length (41.64±0.98/39.76±0.86), root length (33.31±1.71/30.05±0.73), highest dry shoot weight (0.23±0.02/0.24±0.02), and dry root weight (0.36±0.02/0.26±0.02) under both without and with pathogen conditions (Figure 3) (Figure 4). Pseudomonas had a second-highest effect. Carbendazim did not affect. These results are in agreement with the results obtained by Mayo et al. (2015); Okoth et al. (2011); Harman et al. (2004); Jaleed & Baker (1988). As they discussed the increase in the length of root and length of the stem by the treatment of biocontrol agent Trichoderma are the reason for survivability of the seedlings due to its direct effect (such as the production of antibiotics, parasitization of other fungi and competition with the harmful microorganisms) on the plants. Abd El-Moity (1992) observed that Trichoderma harzianum inhibits disease by producing some antifungal substances, i.e. gliotoxin and some growth regulators. These compounds are amphilic, membrane-active surfactants and when applied, prior infection led to stimulate plant resistant and enforce treated plants to produce some metabolites which depress the pathogen and some growth promoters such as indols which increased plant growth generally. Colonization of the roots by Trichoderma asperellum has found to enhance the phosphorus (P) and iron (Fe) availability to plants, which causes significant increase in the dry weight, shoot length and leaf area of the plant (Yedidia et al., 2001). According to Elekhtyar,25 Pseudomonas fluroscens a plant growth promoting rhizobacterium supports the plant growth promotion by two ways i.e directly and indirectly. Directly through synthesis of growth promoting substances or by facilitating the uptake of nutrients and indirectly through preventing the deleterious effect of plant pathogen by the production inhibitory substances or increasing the resistance of plants to pathogens.
From these results, it may be concluded that Trichoderma and even Pseudomonad are potential for the biocontrol of the disease if effective isolates or strains can be applied as they have shown both the inhibitory effect to the pathogen and growth-promoting effect to maize plant. Carbendazim and Trichoderma both can be used for the management of disease if used alternatively as chemical is not found to restrict the growth of Trichoderma completely at low concentration. Thus seed treatment with the biocontrol agent Trichoderma followed with the spray of carbendazim could be the good option for the management of BLSB of maize. Farmers should prioritize, physical, cultural and biological method of disease management and could use carbendazim as the last option.
Authors would like to acknowledge all the supporting hands during the research completion period.
None.
The authors declare there are no conflicts of interest.

©2020 Sagar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.