MOJ
eISSN: 2573-2919


Research Article Volume 7 Issue 3
1Department of Geography, Federal University Birnin kebbi, Nigeria
2Department of Geography, Usmanu Danfodiyo University Sokoto, Nigeria
3Department of Pure and Industrial Chemistry, Federal University Birnin kebbi, Nigeria
4Department of Mathematics, Usmanu Danfodiyo University Sokoto, Nigeria
Correspondence: Saadu Umar Wali, Department of Geography, Federal University Birnin kebbi, P.M.B. 1057 Kebbi State, Nigeria, Tel +2348065555170
Received: April 12, 2022 | Published: May 5, 2022
Citation: Wali SU, Gada MA, Hamisu I, et al. Evaluation of shallow groundwater in Rural Kebbi State, NW Nigeria, using multivariate analysis: implication for groundwater quality management. MOJ Eco Environ Sci. 2022;7(3):65-75. DOI: 10.15406/mojes.2022.07.00249
This study assessed shallow groundwater in rural Kebbi State using Pearson’s Correlation (r), Factor Analysis (FA), and Hierarchical Cluster Analysis (HCA). One hundred (100) shallow groundwater samples were drawn randomly from hand-dug shallow wells in 10 Local Government Areas (LGAs). Physical parameters (pH, temperature, TDS, EC, salinity) were analysed in situ using hand-held metres. Separate water samples were taken to analyse ions (Fe, Zn, K, Mg, Mg, HCO3, Ca). Results revealed that shallow groundwater is lightly acidic, except in Zuru and Fakai LGAs, where an alkaline condition occurred. Correlation analysis revealed that the dissolved solids in shallow aquifers result from natural geological and anthropogenic influences. However, FA had shown that most of the variability in shallow groundwater is influenced by natural geological factors with little evidence from anthropogenic inputs. The HCA categorised shallow groundwater into three groups: those shallow wells having higher concentrations of Zn and Cl with more elevated salinity and temperature; those shallow wells having very low salinity in central Kebbi State, and those shallow aquifers having alkaline waters. Except for higher Fe and Zn concentrations, the shallow aquifers contained water of excellent quality for drinking. Correlation analysis, FA, and HCA present simple statistical tools for assessing the hydrochemistry of groundwater.
Keywords: correlation, factor analysis, hierarchical cluster analysis, shallow aquifers
The assessment of groundwater is an essential activity for rural and urban water management, particularly in semi-arid regions,1–4 because the ionic composition of water has a significant influence on human health.5–7 Using low-quality groundwater for drinking and irrigation affects human health and plant growth and contaminates soil, making it less suitable for agriculture and household uses. Shallow groundwater is the primary source of potable water and is commonly used for household and irrigation uses in rural Nigeria,8,9 particularly in the semi-arid northern areas of Nigeria.10–12 The question of portable and adequate water supply, especially in rural areas, has been unanswered for quite a long time,13–16 leaving the rural dwellers to exploit the shallow groundwater, which can be accessed using simple technologies.
Consequently, water quality is also of concern since most remote communities cannot conduct water analysis.17–19 While shallow groundwater quality in rural areas is poorly known, increasing exploitation of these water sources is driven by Malthusian trends and environmental change.20–22 The uninterrupted increase in the rural population has led to many social, economic, and environmental problems. These problems include excessive shallow groundwater withdrawal for agricultural and domestic uses in arid and semi-arid environments.23–25 Shallow groundwater is the principal industrial, agricultural and domestic water supply.26–28 In some remote communities, shallow groundwater is the principal source of community water supply during the dry season.29–32 It has the advantage of being the inexpensive and reliable source of community water supply,33–36 and this attracts even the more coordinated programs on improving rural water supply to resort to groundwater as the best, inexpensive and dependable source of water supply for community water supplies.37–40
Although shallow groundwater sources are widely used in developing countries,37,41,42s their drinking and agricultural use fitness is poorly investigated. However, shallow groundwater quality varies widely between environments and water sources consequence of geological background and land use.43–46 This erraticism can affect water quality and resultant consequences on human health and wellbeing.47–50 The variability of shallow groundwater composition is primarily influenced by the mineralogy of shallow aquifers51 and human activities (agriculture, industry, etc.), which alter the natural composition of groundwater and consequently its aptness for drinking and other uses, as they can unite to create varied water types that vary in hydrochemistry.
Shallow groundwater has been widely studied.26,36,39,45,50,52–55 Results indicate a need to trace the origin and mechanism of nitrate leakage into the subsurface aquifers of Maiduguri.26 An appraisal of subsurface water quality showed that about half of the shallow groundwater is unsuitable for drinking in Nagpur, India.45 Significant temporal and spatial variations in shallow groundwater temperatures have been revealed in the Lower Heihe River in northwestern China.54 Assessment of health risks of heavy metal contaminations in shallow aquifers indicated that the heavy metal pollution index (HPI) and hazard quotient (HQ) were higher in more cultivated fields.50
Kebbi State is situated in a semi-arid region of West Africa. Apart from human and geological influences on groundwater, the increasing climate vagaries posed a severe challenge to shallow groundwater management. Evaluation of shallow groundwater in the southern of Kebbi State56 revealed that the aquifers are characterised by moderate potassium, calcium, chloride, copper, sodium, and bicarbonates concentrations below the World Health Organization (WHO) and Nigerian Standard for Drinking Water Quality (NSDWQ) reference standards. However, iron, zinc, magnesium, and phosphate concentrations are above the WHO and NSDWQ reference guidelines.57 The study also found that TDS correlates strongly with Cu, Fe, Zn, Mg, and PO4, suggesting that these ions' dissolved solids were derived. However, these findings do not apply to the entire Kebbi State since the geological setting in southern Kebbi State is of a basement complex background. Therefore, this paper aims to assess shallow groundwater across Kebbi State.
The study area
Geographical setting
Kebbi State (Figure 1) is positioned between latitude 100 8’ and 130 15’N and between longitude 30 30´ E. It occupies a land area of about 36,800 km2.58 The climate is characterised by a long dry season and a short but intensive wet season. Annual rainfall is highly variable and decreases in volume from southern to northern parts. The mean maximum temperature is highest in April (400C) and the lowest in December (230C). The mean relative humidity is maximum in August (90%) and is a minimum in December (10-30%). However, there is an overall increase in relative humidity southward.58 The evaporation rate is high, and the state can be classified as having a ustic soil moisture regime.
Hydrogeological condition
The geology is typical of the geological setting of Northwestern Nigeria. It is mainly sedimentary, with some weathered basement complex rock outcrops in the south.59,60 Underlying the sedimentary formations are crystalline rocks of Pre-cretaceous age.61 Groundwater in the upland areas of crystalline rocks of the southern part of Kebbi State is ordinarily accessible in small amounts from fissures or other horizontal separations and the regoliths.59 River Sokoto (Gulbin-Dukku, locally called), a chief tributary of River Niger, essentially drains the study area.62 Despite the potential presented by aquifers in the state (e.g. Gwandu and Illo Formations), many communities are still without improved water supply systems and rely on individual and community hand-dug shallow wells for potable water supply. Figure 2 describes a hydrogeologic cross-section of the Sokoto basin.
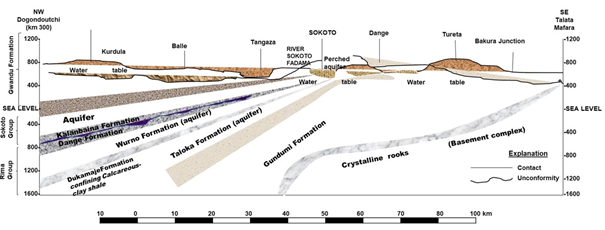
Figure 2 Geohydrologic section of Sokoto basin, NW Nigeria, indicating principal aquifers and confining beds.
Although these hand-dug wells are poorly constructed, their water quality remains poorly known because most rural communities lack the capacity for water testing.19,63 However, the hydrological condition of northern and southern parts of Kebbi State showed that the underlying shallow aquifers are in hydraulic connectivity with surface water bodies.64 Additionally, these aquifers are recharged primarily by infiltering rainwater, making them prone to contamination. Therefore, proper land use practice is required to effectively manage shallow groundwater quality.59
Groundwater sampling and analysis
The study employed onsite field measurements and laboratory analysis (Table 1). Ten (10) Local Government Areas (LGAs) were purposely studied, and ten (10) communities from each LGA were studied at random. Therefore, one hundred (100) water samples were collected and analysed from Hand-dug shallow wells. Before taking water samples, the plastic containers were rinsed twice. First by deionised water and later with water from experimented shallow wells.
Parameters |
Methods |
Description |
Detection limits |
Reference Guidelines |
|
|
|
|
WHO (2011) |
NSDWQ (2007) |
|
Temperature |
Field (DK-MsG01) |
Temp/Salinity-meter |
Ambient |
Ambient |
|
pH |
Field(pHep) |
pH meter |
- |
6.5-8.5 |
6.5-8.5 |
Conductivity |
Field (DIST) |
EC/TDS- meter |
uS/cm |
- |
- |
Salinity |
Field (DK-MsG01) |
Temp/Salinity-meter |
mg/l |
0.5 |
0.5 |
TDS |
Field (DIST) |
EC/TDS- meter |
“ |
1000 |
500 |
AAS |
“ |
0.3 |
0.3 |
||
Zn |
“ |
“ |
“ |
3 |
4 |
K |
“ |
“ |
“ |
500 |
1000 |
Mg |
“ |
“ |
“ |
3 |
4 |
Mg |
“ |
“ |
“ |
200 |
200 |
HCO3 |
“ |
“ |
“ |
250 |
250 |
Ca |
“ |
“ |
“ |
500 |
500 |
Table 1 Parameters, methods, description, detection limit and reference guidelines
Atomic Absorption Spectrometry (AAS) was used for ion analysis.65 AAS is a technique in which free vaporous atoms absorb electromagnetic energy at an explicit wavelength to create a measurable indicator. The immersion of those free riveting atoms in the visual route is proportionate to the immersion indicator. Accordingly, for AAS dimensions, the analyte is initially transformed into vaporous traces, usually by directing heat to a cell (i.e., atomiser). The type of atomiser delineates the two major AAS-based analytic procedures: flame atomic absorption spectrometry (FAAS), which steadily proffers analytical indications and electrothermal atomic absorption spectrometry (ETAAS) conveying logical indicators in a sporadic mode (2 to 4/sample).
In both methods, water (or dissolved) samples are mounted into the analyser as an aerosol in FAAS instance for this analysis or as an apparent low microliter amount in ETAAS. Monochromators founded Ebert, Littrow, and Czerny-Turner schemes are the best standard wavelength pickers applied in AAS. Newly, echelle optics is bonded to marketable AAS appliances. Launching an echelle design connected with a formidable uninterrupted lamp forms momentous capability, for example, the study of the spectral milieu close to the line and simultaneous multi-elemental analysis. The parameters were analysed using AAS (Rs 12.5 Lakh/Piece).
Statistical analysis
The hydrochemical data is summarised and standardised, as presented in Table 2. The data were further analysed using Pearson’s correlation (r), Factor analysis (FA), and hierarchical cluster analysis (HCA). Pearson’s correlations (r) were used to study the relationship between hydrochemical variables (Table 3). Factor analysis was used to measure the interrelationships between the studied hydrochemical parameters by lessening the detailed data to a more interpretable form.66-70 Factor analysis is a multivariate statistical procedure that can be applied to reduce enormous and intricate datasets by identifying a small number of parameters that describe the most significant variation within the original data set. In this paper, FA was carried out on hydrochemical parameters to pinpoint and define the elements that influence the shallow groundwater composition in Kebbi State. The FA was carried out on a subset of 11 chosen hydrochemical parameters (Temperature, EC/Salinity, pH, TDS, K, Ca, Fe, Zn, Mg, Cl, HC03), which characterised the general groundwater composition.
LGAs |
Temperature |
EC |
pH |
Salinity |
TDS |
Fe |
Zn |
K |
Mg |
Cl |
HCO3 |
Ca |
Arewa |
29.5-34.0 |
0.1-28.0 |
4.5-6.4 |
0-0.1 |
10.0-130 |
0.3-2.0 |
1.0-6.4 |
39-156 |
2.0-10.0 |
36.0-3515 |
6.0-183.0 |
3.0-34.0 |
(31.65±0.3) |
(8.62±0.3) |
(5.23±0.5) |
(0.02±0.1) |
(26.0±3.2) |
(1.24±0.1) |
(5.27±0.1) |
(101.4±8) |
(4.8±1.0) |
(562.5±20) |
(110.4±0.8) |
(8.4±.01) |
|
Augie |
28.0-32.9 |
11.0-116 |
0.0-4.1 |
0.0-0.5 |
37.0-1140 |
0.9-3.1 |
4.2-6.3 |
39-177 |
1.0-10.0 |
107.0-244 |
24.0-244.0 |
8.0-37.0 |
(30.03±0.9) |
(39.0±0.1) |
(2.27±0.5) |
(0.15±0.1) |
(296.7±28) |
(2.03±0.0) |
5.56±0.1) |
(54.6±3) |
(5.0±1) |
(114.6±23) |
(114.6±2.2) |
(19.5±0.11) |
|
Bagudo |
24.6-31.1 |
0.0-166 |
2.2-9.2 |
0.0-1 |
70.0-2237 |
0.6-2.3 |
0.7.0-7.5 |
39.0-156 |
1.0-20.0 |
142.0-3408 |
61.0-432.0 |
4.0-111.0 |
(29.03±.0.1) |
(30.34±0.17) |
(6.16±0.5) |
(0.19±0.1) |
(449.7±32) |
(1.54±0.1) |
(5.13±0.1) |
(58.5±1) |
(15.2±1) |
(876.4±19) |
(195.7±1.3) |
(32.8±0.6) |
|
Birnin-kebbi |
27.0-30.0 |
2.0-55.0 |
1.3-5.7 |
0.0-0.6 |
10.0-220 |
3.1-6.4 |
0.3-2.6 |
39.0-177 |
2.0-150 |
4.0-3586 |
61-244 |
4.0-37 |
(28.77±21) |
(12.5±0.21) |
(2.95±) |
(0.09±0.1) |
(56.51±10) |
(4.74±0.1) |
(1.49±0.1) |
(58.5±3) |
(0.62±1) |
(646.8±13) |
(122.0±1.9) |
(13.0±0.8) |
|
Bunza |
29.3-32.1 |
1.0-57.0 |
1.4-3.0 |
0.0-1.0 |
10.0-320 |
0.3-23.0 |
1.6-6.8 |
39.0-273 |
2.0-12.0 |
36.0-6639 |
61.0-427.0 |
5.2-157.0 |
(30.83±0.21) |
(14.0±0.8) |
(1.9±0.5) |
(0.17±0.1) |
(111±1.2) |
(1.25±0.1) |
(4.72±0.1) |
(936.0±4) |
(5.0±1) |
(1100.8±32) |
(201.0±2.0) |
(32.5±1.3) |
|
Fakai |
21.4-31.0 |
3.0-26.0 |
7.1-8.1 |
0.0-0.2 |
50.0-320 |
0.5-2.7 |
2.0-6.3 |
39-78 |
1.0-20.0 |
71.0-188.0 |
61.0-244.0 |
7.0-51.0 |
(28.26±0.7) |
(12.0±0.2) |
(7.64±0.5) |
(0.1±0.1) |
(155.0±12) |
(1.61.0±0.1) |
(4.92±0.1) |
(46.8±1) |
(6.7±1) |
(245.4±14) |
(158.6±.1.2.) |
(22.4±1.0) |
|
Gwandu |
29.0-31.6 |
1.0-167.0 |
4.2-5.4 |
0.0-3.0 |
3.0-1740 |
0.9-2.5 |
0.9-6.6 |
39.0-156 |
1.6-15 |
36.0-9159 |
55.0-244.0 |
5.0-210 |
(30.04±0.5) |
(29.2±1.0) |
(4.78±0.5) |
(0.64±0.1) |
(318.3±20) |
(1.580±0.1) |
(4.61±0.1) |
(50.7±7) |
(5.9±1) |
(1226.2±28) |
(164.1±2) |
(44.7±0.1) |
|
Jega |
29.1-32.5 |
1.0-15 |
6.3-7.1 |
0.0-0.1 |
20.0-1200 |
0.4-2.6 |
3.3-6.3 |
39.0-396 |
1.0-81.0 |
36.0-349.0 |
122.0-366 |
10.0-53 |
(30.59 ±0.31) |
(5.9±0.2) |
(6.71±0.5) |
(0.01±0.1) |
(317±34) |
(1.637±0.1) |
(4.27±0.1) |
(54.6±5) |
(11.6±1) |
(166.0.9) |
(207±3) |
(20.9±0.4) |
|
Maiyama |
28.7-31.6 |
0.3-25 |
3-3.8 |
0.0-1.0 |
10.0-130 |
0.6-2.5 |
3.4-66.0 |
39.0-177 |
1.1-108 |
36.0-3089 |
61.0-305.0 |
5.0-27.0 |
(30.19±1.0) |
(9.03±0.41) |
(3.4±0.5) |
(0.01±0.1) |
(57.0±15) |
(1.507±0.1) |
(5.28±0.01) |
(50.7±3.2) |
(14.51±) |
(435.5±12) |
(140.0±.1.5.) |
(14.4±1.0) |
|
Zuru |
22.3-27.6 |
1.0-40.0 |
6.8-7.8 |
0.0-0.4 |
40.0-240 |
0.6-2.5 |
0.6-2.7 |
39.0-39.0 |
2.0-18.0 |
4.0-497 |
122-549.0 |
1.2-42.0 |
|
(25.04±0.8) |
(10.31±0.3) |
(7.25±0.5) |
(0.09±0.1) |
(129.0±16) |
(1.61±0.1) |
(1.61±0.1) |
(39.0±2) |
(6.5±) |
(132.1±6) |
(195.0±1) |
(16.92±0.2) |
Table 2 The concentration of groundwater quality parameters
Parameter |
Temp |
EC |
pH |
Sal. |
TDS |
Fe |
Zn |
K |
Mg |
Cl |
HCO3 |
Ca |
Temp |
0.86 |
0.155 |
0.382 |
0.986 |
0.567 |
0.028 |
0.035 |
0.859 |
0.311 |
0.428 |
0.834 |
|
EC |
0.064 |
0.412 |
0.982 |
0.035 |
0.947 |
0.323 |
0.638 |
0.994 |
0.491 |
0.668 |
0.119 |
|
pH |
-0.485 |
-0.293 |
0.408 |
0.485 |
0.4 |
0.709 |
0.253 |
0.358 |
0.311 |
0.244 |
0.975 |
|
Salinity |
-0.311 |
0.008 |
0.295 |
0.831 |
0.729 |
0.928 |
0.472 |
0.514 |
0.389 |
0.755 |
0.879 |
|
TDS |
0.006 |
0.669 |
0.25 |
-0.078 |
0.517 |
0.396 |
0.323 |
0.183 |
0.717 |
0.202 |
0.043 |
|
Iron |
-0.207 |
-0.024 |
-0.3 |
0.126 |
-0.233 |
0.039 |
0.601 |
0.132 |
0.89 |
0.269 |
0.379 |
|
Zinc |
0.686 |
0.349 |
-0.135 |
0.033 |
0.302 |
-0.657 |
0.366 |
0.207 |
0.71 |
0.718 |
0.498 |
|
Potassium |
0.667 |
-0.17 |
-0.399 |
-0.258 |
-0.349 |
-0.189 |
0.321 |
0.46 |
0.24 |
0.612 |
0.746 |
|
Magnesium |
0.065 |
-0.003 |
0.326 |
-0.234 |
0.459 |
-0.51 |
0.437 |
-0.264 |
0.839 |
0.206 |
0.668 |
|
Chloride |
0.357 |
0.247 |
-0.357 |
-0.306 |
0.132 |
-0.05 |
0.135 |
0.409 |
-0.074 |
0.652 |
0.027 |
|
Bicarbonate |
-0.283 |
-0.155 |
0.406 |
-0.114 |
0.441 |
-0.387 |
-0.131 |
-0.183 |
0.438 |
0.163 |
0.121 |
|
Calcium |
0.076 |
0.525 |
-0.011 |
-0.056 |
0.647 |
-0.313 |
0.243 |
-0.118 |
0.155 |
0.692 |
0.523 |
|
Table 3 Person’s (r) values in bold show a significant correlation between parameters
Therefore, applying factor scores to HCA is an excellent method for groundwater analysis by simplifying groups into more unrestrained forms, despite the obliviousness of specific theoretically significant hydrochemical data. However, this method does not account for data validity and disconnected values and neglects credible and possible connections or resemblances (statistical meddling) between variables, which are somewhat clear in most hydrochemical data.66–70 As a result, the output would have been inexorable to manipulation and not be deemed as complete hydrochemical momentous data. Therefore, in this paper, only raw data were included in the HCA, where full scrutiny of each cluster is possible, and accurate (without modification) condition of groundwater composition could be characterised.
Physicochemical characteristics of groundwater
Table 2 summarises groundwater parameters (range, mean and standard error). The pH concentration varied in the study area. Mean pH was above 7 in Zuru and Fakai LGAs, indicative of alkaline conditions. The two LGAs are in the basement complex section of southern Kebbi State. Therefore, the observed alkaline condition perhaps results from the region’s geology mainly comprises crystalline rocks. High pH in aquifers can be linked to the ion exchange process.74,75 It accelerates the evolution from calcium to soda through subsurface water metamorphisation with a successive increase in the carbonate substance and alkalinity of aquifers.76
However, a high pH level of 11-12 was recorded in Paju City consequence of shallow groundwater pollution caused by improper discarding of road constructions litter on the surface at the study location.77 Arewa and Jega LGAs have a mean pH of 6.5, indicating a neutral condition. The seventeen LGAs have pH values of less than 5 displaying acidic conditions. Groundwater acidity has been reported from various parts of the world.78–80 Aerosol particles and acid gases are incorporated into cloud water and rainwater and deposited by rainfall or by tumultuous mixing and gravitational landing on vegetation, soil, unprotected water, and streams/rivers. The existence of rain tends to be correlated to increased anthropological emission of gaseous pollutants into the atmosphere, mainly sulfur and nitrogen oxides.80
The most acidic water has occurred in the eastern section of the Sokoto basin and the south.59 Just after Sokoto town, at Dange pH is 5.1 at Borehole GSN 3512, 3.7 at Borehole GSN 3520, and it is 3.7 at 177km at Borehole GSN 3519 along the Sokoto-Gusau road. Groundwater in this vicinity also has low TDS (28-79mg/1). The major anion is SO4, which might have been derived from the oxidation of pyrites.59 This process perhaps accounts for the acidity of the water in the western Sokoto basin. The temperature varied between the studied LGAs. The mean groundwater temperature is lowest in Zuru and highest in Bunza. Elevated groundwater temperatures are strongly associated with increased chemical reactions in aquifers. A temperature increase by 10oC can lead to the doubling of chemical reactions in aquifers.81
Mean EC concentration is highest in Gwandu and Augie LGAs, indicating that the two LGAs have high dissolved rock mineral contents in shallow groundwater. Higher groundwater temperatures in the two LGAs could account for higher EC values.82,83 There tends to be an upward trend between elevated temperature and EC level in aquifers.81 Mean salinity values were lowest in Jega and Maiyama LGAs. At the same time, Gwandu LGA has the highest mean salinity, which exceeds WHO and NSDWQ reference guidelines (0.5mgl). The observed high salinity can be derived from rock minerals and land use (irrigation), which is well pronounced in the Gwandu LGA. High salinity in subsurface water is derived from natural and anthropological processes.84–89 Most of the irrigated fields in Gwandu LGA are underlain by a very shallow groundwater table (often less than 2 meters below the ground surface). The continuous application of agrochemicals (especially chemical fertiliser) in these fields alters the chemical composition of shallow aquifers, which is hardly unconnected with high EC/salinity and TDS level in the area. Human activities, especially agriculture, posed a severe threat to groundwater quality.72,87,90–92
Figure 3 shows the relative concentrations of groundwater parameters. Rises in pH accompanied an increase in groundwater temperature while both parameters decreased with an increased salinity level. This was not the expected outcome since EC values in groundwater show an upward trend with rising temperature and TDS levels (EPA, 2001). Similarly, the latter rises with a rise in EC, Zinc, and iron. However, an increase in pH level was also accompanied by an increase in salinity and EC levels. As a result, mean total dissolved solids (TDS) are within WHO and NSDWQ reference guidelines (Table 1).

Figure 3 The relative concentration of physicochemical parameters. Note: all concentrations are in mg/l except conductivity (µS/cm) and pH (unit).
The TDS is widely used in water quality analysis as it shows the number of dissolved rock materials in groundwater, which linger as a residue after water evaporation from the sample.93,94 The general outlook of the TDS concentrations in Kebbi State indicates low concentrations of dissolved solids in shallow groundwater. Results are concurrent with previous investigations on the hydrochemistry of the Sokoto basin.59,95,96 Lower TDS intensities (<500mg/l), is exceptionally essential.97 Based on TDS concentrations, shallow aquifers are appropriate for drinking. Iron concentration generally fell outside WHO and NSDWQ reference guidelines (Table 1) (Table 2). This finding concurred with the existing literature relating to the hydrochemistry of the Sokoto basin.53,59 Mineral iron is widely distributed on earth and is obtained from different sources.98,99 Except for high iron concentrations, groundwater in Kebbi State is generally fit for drinking based on anion chemistry. An elevated concentration of iron is quite harmful to aquatic life.100–102 Typically there is no effect on human health; instead, the problems are primarily aesthetic.81 Except for Birnin kebbi and Zuru LGAs, the mean zinc concentration fell outside the WHO and NSDWQ reference guidelines. Figure 3 shows an upward trend between Zinc and iron concentrations, even though the former showed a downward trend with increased TDS. Therefore, most of the observed TDS values are derived from iron.
Zinc concentration was higher in Arewa, Augie, Bagudo, and Maiyama LGAs. Mean zinc values in these LGAs exceed WHO and NSDWQ reference guidelines even though zinc is vital to a man if consumed in significant quantities.81 The noxiousness of zinc to aquatic life is (as with copper) contingent on the hardness of the water, which declines with increasing hardness.81 Potassium concentration is within the WHO and NSDWQ reference guidelines. Figure 4 shows the relative potassium, magnesium, chloride, bicarbonates, and calcium concentration. The observed consistency in potassium values results from homogeneity in soil chemistry since potassium is fixed in soils and is not merely seeped out.103–105
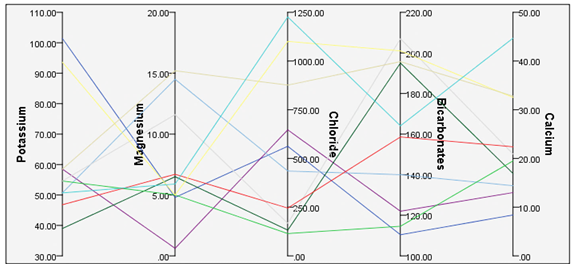
Figure 4 The relative concentration of groundwater parameters. Note: all concentrations are in mg/l.
Magnesium is high and exceeds WHO and NSDWQ reference guidelines. Undue intake has not been related to any serious health threat, only that its concentrations are very significant when considered with sulfate.81 Alkali metals such as Ca joint with Mg are responsible for groundwater hardness. Magnesium is a relatively common reducing element. Temperature changes can impact aquifers by rising oxygen and increasing the reduction process. Also, Mg can react with water vapour to produce hydrogen gas or magnesium hydroxide, as defined in equation 1. Magnesium constitutes a second major component of hardness (CaCO3). Dolomite and magnesium carbonate are primary sources of Mg in aquifers.106–108
Mg(s)+2H2 O(g)→Mg(OH)2 (aq)+H2 (g) (1)
Mean calcium is high in Bagudo and Bunza LGAs. Typically, Ca concentration is low in Kebbi State. The importance of Ca in the hydrochemical analysis also relates to hardness. Calcium is found naturally in various environmental settings and occurs widely in groundwater aquifers.109–111 It is an integral component of coral and is found in high concentrations (400 mg/l) of brine. In lime regions, Ca attains 100 mg/l. Elementary Ca at normal temperature reacts with water, based on the reaction process indicated by equation 2.
Ca(s)+2H2O(g)→Ca(OH)2 (aq)+H2 (g) (2)
Dissolved calcium hydroxide forms soda and hydrogen gas. It typically occurs when CO2 is freed, resulting in the development of carbonic acid, affecting Ca compounds. The carbon weathering and total reactions are defined in equations 3 and 4. Consequently, calcium hydrogen carbonate is produced.
H2O+CO2→ H2CO3 and CaCO3+H2 CO3→CaH(CO3)2 (3)
CaCO3 (s)+CO2 (g)+2H2 (I)→Ca(aq)+2HCO3 (aq) (4)
Mean chloride concentration in Arewa, Bagudo, Bunza, Gwandu, and Maiyama exceeds WHO and NSDWQ reference guidelines (Table 2). As shown in Figure 4, a rise in chloride concentration accompanied rising Mg, HCO3, and Ca levels. However, these ions are geologically unrelated to chloride. Chloride levels can be as high as 70 mg/l under dry season discharges from industrial and municipal sewage. High ingestion presents no health risks. Though, a higher Cl level in irrigation water might render it unsuitable for irrigation.112–114
Combined with Ca and Mg, Bicarbonates develop carbonate hardness.115–117 Bicarbonate and carbonate ions merged with Ca, or Mg will precipitate as calcium carbonate (CaCO3) or magnesium carbonate (MgCO3) when the soil solution distillates under dry environments.45 Consequently, the concentration of Ca and Mg declines relative to sodium, and the SAR index will increase.118 This causes an alkalising effect and raises the pH level. Thus, when a water analysis shows a high pH level, it can signify high content of carbonate and bicarbonate ions.118 Bicarbonate is another alternative measure of the sodium content concerning Ca and Mg. Bicarbonate ion is formed consequential of bonding positively charged ion with negatively charged O2 molecules, producing an ionic fused. Bicarbonates usually are soluble in water. Mean bicarbonate in the study area was greater in Bunza and Jega LGAs. The overall bicarbonate concentration in Kebbi State is within WHO and NSDWQ reference guidelines.
Statistical application
Correlation analysis
Table 3 shows the correlations between hydrochemical parameters. Temperature correlates significantly with EC. This was an expected outcome and concurred with most findings in the literature.119–121 Conductivity and salinity correlate significantly. This is also an expected outcome since the two parameters are often used interchangeably, as, in most literature, EC is used as an alternative measure of salinity.122,123 EC correlates significantly with Fe, K, HCO3, Ca, and Mg. Thus, the dissolved solids are derived from these ions. pH was significantly correlated with Ca. In groundwater, pH and Ca tend to be correlated; the former can be altered by calcium hydroxide and calcium carbonate. A reduction in pH consequent to precipitation of calcium carbonate has been noticed.124
Salinity correlates significantly with all the analysed parameters, except K and Cl, indicative of the lesser contribution of these elements to salinity in the study area. TDS correlates significantly only with Fe and Cl, suggesting that the dissolved solids in shallow aquifers are derived from these ions. Magnesium was associated considerably with Ca and Cl. A significant correlation between Ca and Mg suggests the same source, calcite rocks.118 Chloride correlates significantly with HCO3, meaning the same source, a natural geogenic source. Chloride is inherently found in aquifers since it is increasingly derived from anthropogenic sources (agrochemicals, industrial and urban sewage) and mineral lodes.125,126 Though chloride (Cl) is a stable ion, it is naturally combined with sodium and sporadically with Ca, Mg, and K. Natural aquifers rarely contain Cl above 50 mg/l, as any significant rise may lead to suspicion from anthropogenic inputs.
Bicarbonate is a transitional form within the dehydronation of carbonic acid. It serves an essential role biochemically in the buffering system of pH .127-129 Bicarbonate is the conjugate acid of CO3, and the conjugate is based on the acid H2CO3 (carbonic acid). The carbonate reaction is defined in equations 5 and 6:
CO32-+2H2O↔HCO3-+H2O+OH-↔H2 CO3+2OH-(5)
H2CO3+2H2O↔HCO3-+H2O↔CO32-+2H3O (6)
The bicarbonate ion is produced by bonding positively charged ions with negatively charged O2 molecules, creating an ionic composite. Bicarbonates are typically soluble in water.
Factor analysis
Factor analysis (FA) is a robust statistical technique that aids the explanation of groundwater composition.130–133 Factor analysis can be applied to reorganise data to describe better the characteristics of the environmental factors that produced the observed groundwater composition. Therefore, a set of factors (Table 4) was extracted to explain the interrelations between groundwater elements. A total of five factors were extracted. The five factors put together explained 88.20% of the total variance. Factor 1 accounts for 27.75% of the variance and is inferred as correlating primarily to the salinisation of the shallow groundwater from the dissolution of rock minerals.134,135 This factor has high positive loadings (≥0.65) on Zn, Mg, Ca, TDS, and EC. In addition, the major cation exchange elements Ca, and Mg correlate positively, which is also indicative of the effect of the dissolution of rock minerals.
Parameter |
Factor 1 |
Factor 2 |
Factor 3 |
Factor 4 |
Factor 5 |
Temp |
0.402 |
-0.78 |
-0.26 |
0.111 |
-0.044 |
EC |
0.525 |
-0.053 |
0.62 |
0.495 |
-0.12 |
pH |
-0.004 |
0.778 |
-0.355 |
-0.024 |
0.237 |
Sal |
-0.277 |
0.308 |
0.082 |
0.505 |
0.695 |
TDS |
0.74 |
0.414 |
0.292 |
0.203 |
-0.177 |
Fe |
-0.641 |
-0.102 |
0.594 |
-0.015 |
-0.214 |
Zn |
0.675 |
-0.336 |
-0.391 |
0.505 |
0.119 |
K |
0.094 |
-0.798 |
-0.288 |
-0.244 |
0.255 |
Mg |
0.56 |
0.386 |
-0.479 |
0.039 |
-0.387 |
Cl |
0.513 |
-0.426 |
0.393 |
-0.481 |
0.227 |
HCO3 |
0.448 |
0.57 |
-0.09 |
-0.573 |
0.115 |
Ca |
0.785 |
0.136 |
0.461 |
-0.206 |
0.271 |
Eigenvalues |
3.33 |
2.917 |
1.864 |
1.473 |
1.001 |
% of Variance |
27.747 |
24.306 |
15.532 |
12.275 |
8.34 |
Cumulative % |
27.747 |
52.052 |
67.584 |
79.859 |
88.199 |
Table 4 Factor analysis (Varimax rotation), values in bold signify high loadings
Hierarchical cluster analysis
Hierarchical cluster analysis is a widely used technique in groundwater analysis since it can differentiate groundwater composition.71–73,76,141–145 By applying HCA, the sampling LGAs with comparable groundwater hydrochemistry can be merged into a single cluster. The graphical assemblies of the clustering process are illustrated in Figure 5. Based on the dendrogram, sampling LGAs with matches are collected into three different sets. The first cluster (group 1) sampling LGAs: Arewa, Birnin kebbi, Bunza, Maiyama, Bagudo, and Gwandu. Groundwater composition in these LGAs is characterised by higher chloride, zinc, and higher salinity and temperature levels. The high temperature in aquifers can be related to external influences (climatic factors) or geologic effects.146,147
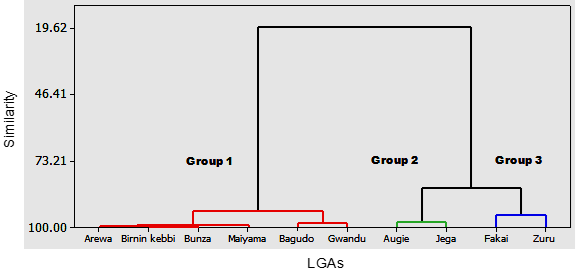
Figure 5 A dendrogram produced from cluster analysis based on the sampling LGAs to identify the significant groundwater characteristics in Kebbi State.
Group 2 comprises sampling LGAs: Augie and Jega. Jega LGA had the lowest salinity. Several factors such as vegetation, quality of recharge and groundwater level depths, and aridity account for salinity variability in groundwater.148–150 Sampling LGAs Fakai and Zuru encompass the last group. Groundwater composition in these LGAs is alkaline. The salinisation/alkalinisation of aquifers is controlled by many factors.151–154 Overall, three types of shallow aquifers are noticeable in Kebbi State: those shallow aquifers with higher concentrations of Zn and Cl, salinity and temperature in northern Kebbi State; those shallow aquifers with very low salinity in central Kebbi State; and those shallow aquifers with alkaline waters in southern Kebbi State.
The implication for groundwater management
This study showed that shallow groundwater in Kebbi State is fit for human consumption. Rock weathering appeared to be the primary mechanism controlling the hydrochemistry of groundwater. A multivariate analysis approach can be applied to explain the variability of hydrochemistry of shallow groundwater. It can reveal natural geogenic and anthropogenic alteration of groundwater chemistry that may have severe consequences on humans and the entire ecosystem services that rely on shallow aquifers.155 Over the last 3-4 decades, many countries have employed direct and indirect approaches to groundwater management.156
Increasing human activities with corresponding changes in land use has made groundwater quality management an arduous task.157–159 Consequently, multivariate analysis can classify groundwater sources and detect possible pollution from anthropogenic activities.160–162 Although accumulating groundwater contaminants are rising in shallow aquifers consequence of human activities, increased demand for shallow groundwater for irrigated farming is making groundwater quality management increasingly tricky.163,164 It also requires innovative water quality management approaches lacking in most developing countries. Several pollutant modelling approaches exist for assessing pollution in aquifers. However, most of these approaches are complex and require a large amount of data which may be lacking in places like Kebbi State. While multivariate analysis (MA) can be used to study groundwater pollution, MA cannot quantify the actual risk exposure while using polluted aquifers for domestic, industrial, or agricultural purposes. Therefore, many recommendations for enhancing the quality of groundwater aquifers are needed. These include the application of water quality modelling approaches, the formation of pollution monitoring agencies, policies for ensuring sustainable land use and water resources protection.
The literature is undivided on the significance of water quality and the need to understand the physical and chemical composition of any water meant for drinking, especially in developing countries like Nigeria. This study has reported our recent efforts to represent better the status of rural water quality from remote communities of Kebbi State. Understanding the hydrochemistry of groundwater from shallow aquifers is essential for the success of improved water supply programs which are in progress in many parts of the state. Findings from this study lead to the following remark:
The shallow aquifers contained water of good quality based on the analysed parameters. This finding is helpful since it revealed the status of superficial groundwater quality in Kebbi State. Results can be used as future reference material for water quality management. Since there is insignificant proof of shallow groundwater pollution, this finding can be used as a baseline reference material for future analysis of the hydrochemistry of shallow aquifers. It can also be used as a guiding principle for future policy on rural water supply. Correlation analysis, FA and HCA provide simple tools for assessing groundwater. We hope that this paper will stimulate other scholars to use a comparable method in a future water quality study.
This study was financed by the Tertiary Education Trust Fund (TETFund). We also thank all the anonymous contributors.
None.
Authors declare that there is no conflict of interest.

©2022 Wali, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.