MOJ
eISSN: 2573-2919


Research Article Volume 6 Issue 6
1Soil Science Department, Faculty of Agriculture, Cairo University, Egypt
2Plant Researches Department, Nuclear Research Center, Atomic Energy Authority, Egypt
3Agronomy Department, Faculty of Agriculture, Cairo University, Egypt
4Health Affairs Directorate, Damietta, Ministry of Health, Egypt
5American International University-Bangladesh, Bangladesh
Correspondence: Badawy SH, Soil Science Department, Faculty of Agriculture, Cairo University, Egypt
Received: October 31, 2021 | Published: November 23, 2021
Citation: Badawy SH , El-Motaium RA, El-Sayed MA, et al. Environmental impact assessment of Egyptian Damietta governorate soils contamination with cadmium. MOJ Eco Environ Sci. 2021;6(6):216-228. DOI: 10.15406/mojes.2021.06.00236
Soil parental materials and anthropogenic activities are the sources of increasing Cd in soils and enrichment in edible parts of plants and creating hazards to animals and human. Thus, it is an important issue to estimate the levels of Cd in soils; straw and grains of rice and wheat plants grown in the soils contaminate with Cd and evaluates human health risk. In surface soil, total Cd (899±497µg kg-1) and Diethylene Triamine Penta Acetate (DTPA) extractable Cd (16.41±13.83µg kg-1) slightly higher by 1.02±0.20 and 1.31±0.45 folds than the subsurface layers, respectively. The DTPA extractable Cd concentration is significantly increased linearly with increasing total soil Cd (r=0.90). Positive significant correlation was found between soils DTPA extractable Cd and soil organic matter (OM) content (r=0.95), while significant negative correlation for both CaCO3 content (r=-0.92) and pH (r=-0.94). In rice Cd concentrations of straw (374±156µg kg-1) and grains (35±16µg kg-1) are significantly correlated with total soil Cd (r=0.89, r=0.86) and DTPA extractable Cd (r=0.84, r=0.74), respectively. Whereas, rice grains Cd is increased with increasing straw Cd (r=0.98). Wheat Cd concentrations of straw (194±71µg kg-1) and grains (18±13µg kg-1) are significantly correlated with total soil Cd (r=0.90, r=0.96) and DTPA extractable Cd (r = 0.91, r=0.95), respectively. Wheat grains Cd is increased with increasing straw Cd (r=0.95). The Cd Transfer factors (TF) as an average is higher in rice grains (0.04±0.014) than wheat grains (0.021±0.016). However, Cd concentrations in the wheat and rice grains are lower than the both EU and WHO/FAO permissible limits (0.24mg kg-1 and 0.4mg kg-1 dry wt., respectively) and so far no potential human health risk is concluded yet.
Keywords: cadmium, human risk assessment, rice, wheat, pollution, toxicity
TF, For example; OM, organic matter; DTPA, diethylene triamine penta acetate
Cadmium content of non-polluted soils is largely dependent on the rocks from which soil parent materials was derivative and the process of weathering to which the soil-forming materials have been subjected. While soils contamination are produced from anthropogenic Cd sources include industrial waste, sewage effluent, mining, atmospheric deposition of combustion emissions1,2 and from the application of phosphorus fertilizer.3 Thus, Cd is the metal that attracts the most attention in soil science and plant nutrition because it’s potential poisoning for humans and their toxicity problems of the environment.4
Numerous studies are conducted in several countries to estimate Cd in soils which cleared those high levels of Cd in soils may be inhibit crop production, or increase Cd uptake without reduction in yield.5 In alluvial soils of Delta, Egypt, total Cd concentration ranges from 0.7mg kg-1 to 1.4mg kg-1.6 Pendias and Pendias7 shows that the average Cd contents of soils lie between 0.06mg kg-1 and 1.1mg kg-1. In the UK, 5km grid survey of soils (5692 samples) showed an average Cd concentration 0.7mg kg-1.8 Holmgren et al.9 recorded the average Cd concentrations in agricultural soils USA (3045 samples were found to be 0.27mg kg-1). However, soils which currently receive sewage sludge will be expected to contain less Cd than the mandatory limits, which are 20mg kg-1 for the US and 3mg kg-1 or below for countries of the EU.10 The USEPA,11 WHO/FAO,12 Chauhan and chauhan13 and Chaoua et al.14 recorded that the safe limits of Cd in soils ranges from 3mg kg-1 to 6mg kg-1. In China, soil total Cd concentration ranges from 1.69mg kg-1 to 2.35mg kg-1.15
Cadmium is one of the most mobile heavy metals in the environment.16 However, the fate of Cd in the soil depends mainly on the total soil Cd concentration17 and the relative balance between sorption, leaching and plant uptake. These processes are strongly affected by soil properties such as pH, contents of organic matter, clay, and active carbonates.16,18,19
Rice and wheat grains Cd accumulation from polluted soil are a great concern due to inadvertently important source of dietary Cd intake by people.20–22 Cadmium concentration ranges in wheat have been reported by previous studies around the world. In china, from 012mg kg-1 to 0.24mg kg-1,15,23 Canada, 0.25mg kg-1,24 Australia, from 0.003mg kg-1 to 0.03mg kg-1.25 Several studies on rice carried out and have found slightly lower or similar levels of Cd, ranges between <0.006mg kg-1 and 0.09mg kg-1.26–28 The maximum permissible limit in polished rice grain as safety of food and human health has set 0.4mg Cd kg−1.29 The tolerable daily intake per person of Cd is one μg Cd kg-1 body weight, an equivalent to a daily intake of 70μg Cd for an adult of 70kg.30 The more recent western European diet studies show that the average dietary Cd intake by adults in Western Europe ranges from 7 to 32μg Cd day-1 with the lowest values shown in the Scandinavian countries and the highest values in the Mediterranean countries. However, in Japan the average intake is generally 40 to 50μg Cd day-1 but may be much higher in cadmium-polluted areas.11
Agriculture is one of the main activities of Damietta governorate, Egypt; in summer season rice crop represents 73.2%, however; in winter season wheat crop represents 21.3% of total cultivated area.31 The main irrigation source of study area is Damietta Nile branch. In addition to the quantity of agricultural drainage water, industrial wastewater and treatment of domestic wastewater, which suffered from intensive pollution, flowing back into the Nile river and becoming available again as mixed polluted water.32–34 Moreover, the application of phosphate fertilizer (either in the form of Triple Super phosphate or Calcium phosphate) is required during soil preparation for crops growing and approximately 24,075 metric tons is used each year.31 These resources are expected to contribute to the accumulation of cadmium in soil and plants. Therefore, our study aimed to quantify the concentration of Cd in soils and crops cultivated in study area and evaluate the possible human health risk caused by the daily intake through contaminated rice and wheat grains using standard tolerable daily intake.
Study area
The study was conducted in Damietta governorate (Egypt), located at the Mediterranean Sea to the northeast of the Nile-Delta (Figure 1). It is lies between these coordinates 31o 28’ 29” to 32o 03’32” E and 31o 09’ 28” to 31o 31’ 45” N. It covers an area of about1029 km2 and representing 4.7% of the Delta-region and about 1.22% of Egypt total area.35 Soil classification order was Entisols,36 with three sub orders: Typic torrifluvents; Typic torripsamments and Typic psamaquents according to soil survey staff (1975). Agricultural land constitutes the majority of Damietta area which covered approximately 50,000 hectare (ha), which encompasses 4 districts; El-Zarqa (5,500ha.), Faraskur (9,500ha.), Kafr Saad (26,000 ha.), and Damietta (9,000ha.). Wheat, maize, cotton, rice, potatoes, lemons, grapes, and tomatoes are famous for growing.31 According to the Central Laboratory of Agricultural Climate (www.clac.edu.eg) of the Ministry of Agriculture, climate of Damietta is generally Mediterranean, where dry summer predominates with mild dry winter. Annual winter temperatures fall to 13˚C in January and rise to 26˚C in August with a mean annual temperature of 20˚C. Precipitation (P) is generally low and does not exceed 125mm/y. Due to its occurrence close to the Mediterranean Sea, the humidity is generally high with maximum value during summer months (up to 76%).
Soil sampling and analysis
The total one hundred forty-two composite soil samples (12 subsamples one kg each) were taken; seventy-one samples from surface (0 to 30cm) and seventy-one samples subsurface (30 to 60cm) to represent agricultural area of four districts (10 surface and 10 subsurface for El-Zarqa, 17 surface and 17 subsurface Faraskur, 29 surface and 29 subsurface Kafr Saad, and 15 surface and 15 subsurface Damietta). Locations of the studied sites were identified using a "GPS" (Model German; Figure 2). Soil samples were air-dried, grounded using wooden mortar, passed through a 2mm sieve before use and prepared for analyses. The particle size distribution (sand, silt and clay), soil texture was performed using the pipette method,37 pH, EC, total CaCO3, organic matter (OM), according to standard methods outlined by Jackson38 and Diethylene Triamine Penta Acetate (DTPA) extractable Cd according to method of Lindsay and Norvell.39 Total Cd was measured using Aqua regia extraction methods40 and Cd concentrations in extractions were measured by Graphite Furnace-Atomic Absorption Spectrophotometer (GF-AAS), Shimadzu 6800, Japan. Physical and chemical characteristics of the investigated soils are presented in Table 2.
Irrigation water sampling and analysis
Twenty irrigation water samples were collected from the irrigation canal sources of study area to represent 4 districts; El-Zarqa (4), Faraskur (4), Kafr Saad (7) and Damietta (5). Locations of the irrigation water samples sites were identified using a GPS (Model German; Figure 3). From each location 10 liter water samples were collected in clean polyethylene bottles and stored at 40C until analyses. Water pH, electrical conductivity (EC), soluble cations and anions were measured according to standard methods.41 Soluble Cd in water samples were directly determined after the filtration. The total Cd concentrations was done by adding 2ml of concentrated HNO3 and 5ml of concentrated HCl to a 100ml aliquot of collected water sample. The solution was covered with a watch glass and heated at 95˚C till volume reduced to 15ml before being allowed to cool. Thereafter, the final volume was adjusted to 25ml with reagent water and replicates were processed on a routine basis to determine precision. The concentrations of Cd in the filtrate of water were estimated using the GF-AAS. Collected irrigation water analysis presented in Table 1.
Locations** |
EC |
pH |
Cd concentration (µg L-1) |
|
(dS/m) |
soluble |
total |
||
A |
0.49±0.07 |
7.63±0.28 |
n.d. |
10±2.7 |
B |
0.71±0.28 |
7.73±0.41 |
n.d. |
12±4.7 |
C |
0.73±0.33 |
7.75±0.14 |
15±5.4 |
28±7.1 |
D |
1.79±2.08 |
7.72±0.15 |
22±7.4 |
31±10.1 |
***Allowed Limits in irrigation water |
< 0.7 |
6.5 - 8.4 |
10 |
|
Table 1 Electrical conductivity (EC), pH and Cd concentration of collected irrigation water from different districts
*n.d, not detected; **A, Al Zarqa; B, Faraskur; C, Kafr Saad; D, Damietta
*** FAO (2017).
Locations* |
Soil depth |
Soil properties (Mean ± Sd) |
||||
(cm) |
Particles size distribution (%) |
|||||
|
C. Sand |
F. Sand |
Silt |
Clay |
Texture** |
|
A |
0-30 |
2.13±0.67 |
11.4±1.25 |
38.0±3.62 |
49.2±4.48 |
C. |
30-60 |
2.15±0.14 |
4.62±2.00 |
45.0±4.19 |
51.7±2.10 |
Si. C. |
|
B |
0-30 |
2.34±1.34 |
7.41±1.34 |
38.6±1.40 |
53.2±1.61 |
C. |
30-60 |
4.71±0.52 |
11.3±1.41 |
35.6±2.30 |
49.8±3.32 |
C. |
|
C |
0-30 |
21.9±29.2 |
14.7±5.80 |
23.5±12.6 |
37.4±21.0 |
C.L |
30-60 |
24.5±40.1 |
12.4±6.98 |
22.1±14.9 |
41.9±32.4 |
Si .C. |
|
D |
0-30 |
23.7±26.9 |
15.5±2.50 |
23.9±12.6 |
40.2±23.0 |
C. |
30-60 |
28.2±29.1 |
13.9±4.15 |
22.2±14.9 |
36.3±21.9 |
C.L. |
|
Chemical properties |
||||||
OM (%) |
pH (1:2.5) |
CaCO3 (%) |
EC (dS/m) |
|
||
A |
0-30 |
1.80±0.65 |
8.06±0.15 |
2.77±0.59 |
2.13±0.46 |
|
30-60 |
1.05±0.37 |
8.18±0.21 |
2.54±1.05 |
2.47±0.76 |
||
B |
0-30 |
2.00±0.23 |
8.23±0.16 |
1.96±0.69 |
3.00±1.47 |
|
30-60 |
1.39±0.19 |
8.23±0.16 |
1.96±0.69 |
3.37±1.40 |
||
C |
0-30 |
2.23±0.64 |
8.10±0.25 |
2.58±1.19 |
3.26±1.90 |
|
30-60 |
2.24±0.73 |
8.27±0.19 |
2.68±1.28 |
3.04±1.45 |
||
D |
0-30 |
1.72±0.18 |
7.90±0.14 |
1.86±1.26 |
3.17±2.67 |
|
|
30-60 |
1.14±0.32 |
8.00±0.18 |
1.88±1.35 |
3.40±2.78 |
|
Table 2 Mean levels, and standard deviation (±Sd) of soil physical and chemical properties in the different districts
*A, Al Zarqa; B, Faraskur; C, Kafr Saad; D, Damietta
** Si. C, silty clay; CL, clay loam; C, clay.
Plant sampling and analysis
A composite sample of rice and wheat plants (50 plants each) was carefully harvest from the same locations where soil samples were taken at maturity stage by hand (Figure 2). Samples washed with tap water and deionized water three times to remove any adhered soil particles, dried at 80˚C for 3 days to a constant weight and grounded before analysis. Samples were separated to straw and grains. The oven-dried plant materials were ground using stainless steel mill and kept for chemical analysis. The ground oven dried plant was subjected to digestion using mixture of acids (HNO3-H2SO4-HCLO4) as described by Jackson.38 Concentrations of Cd were measured in the digest solution by GF-AAS.
Enrichment coefficient (EC) and transfer factor (TF)
Enrichment coefficient and transfer factor can be used to evaluate the ability of plant to accumulate the heavy metal from soil and preferential partitioning in plant parts.23,42,43 Enrichment coefficient (EC) of Cd was calculated as follows: EC=Cd concentration in plant/Cd concentration (total) in soil. The TF of cadmium from straw to grains was calculated as a ratio of Cd concentration in grains divide by Cd concentration in straw.
Health Risk Index (HRI)
The health risk index is calculated by dividing daily intake from the obtained data for rice and wheat grains of Cd by reference oral dose.30 This index represents the harmful to people in which consume food contaminated with Cd. Meanwhile, if the value of HRI is less than 1, people will safe to eat those kinds of food.44
Statistical analyses
Statistical analyses of variance were carried out on the obtained results for all the studied parameters according to the procedure described by Snedecor and Cochron.45 Factorial experiment arranged in randomized complete block design with two factors (soil depths and locations) were used for analysis all data with unequal numbers of composite samples for each location for Al Zarqa, Faraskur, Kafr Saad and Damietta, respectively by used SAS 9.4 program. Duncan’s new multiple range test (DMRT) was applied to detect the significant differences between tested treatments means.46 The relationship among different traits, simple correlation coefficient measures the strength and directions of association between two variables are calculated and the statistical significance of correlations is preceded according to Gomez and Gomez.47
Cadmium in soils
Soil total Cd content
Results of Cd concentrations in the surface and subsurface soil samples collected from different district of Damietta governorate (Figure 4). Generally, the concentrations of the total Cd vary from district to another district and ranges from low for uncontaminated soils too high for soils receiving historically large quantities of Cd through agricultural or industrial activities. The Cd concentrations range for all studied soil samples ranges from 171 to 2235µg kg-1 with an average of 899±497µg kg-1 in the surface layer and from 128 to 1720µg kg-1 with an average 878±430µg kg-1 in the subsurface layers. In Egypt, Rashad et al.6 found that the normal level of Cd in alluvial soils of Delta ranges from 700 to 1400µg kg-1 for total content. The average Cd concentration of all the studied soils in the surface layers from 1.08 to 1.30 within an average 1.02±0.20 fold than the subsurface layers, however, the data showed no significant difference between them (Table 3). The decreases in subsurface soil Cd contents relatively not high comparing with surface layers, may be due to high mobility of Cd with soil depth and well agreement with recent study2 they mentioned that Cd is one of the most mobile heavy metals in the environment.

Figure 4 Minimum, maximum and average of soil total cadmium concentrations in the different districts.
Depth (cm) |
Location* |
Soil Cd concentration** (µg kg-1) |
|||||
Total |
DTPA extractable |
||||||
Mean ** |
±SD |
CV% |
Mean |
±SD |
CV% |
||
0 - 30 |
899 a |
497 |
55.3 |
16.4 a |
13.83 |
84.3 |
|
30 - 60 |
874 a |
433 |
49.6 |
12.6 b |
8.34 |
66.4 |
|
A |
566 c |
56 |
9.9 |
8.6 c |
1.02 |
11.8 |
|
B |
512 c |
154 |
30.1 |
7.8 c |
2.44 |
31.2 |
|
C |
1154 a |
492 |
42.7 |
20.0 a |
14.73 |
73.8 |
|
D |
1008 b |
388 |
38.6 |
15.4 b |
8.8 |
57.2 |
|
0 - 30 |
A |
582 c |
62.5 |
10.8 |
8.6 d |
1.17 |
13.6 |
B |
519 c |
185 |
35.8 |
7.9 d |
2.51 |
32.4 |
|
C |
1175 a |
542 |
46.2 |
23.1 a |
17.23 |
74.7 |
|
D |
1012 b |
432 |
42.9 |
18.6 b |
11.13 |
60 |
|
30 - 60 |
A |
550.2 c |
46.3 |
8.4 |
8.5 d |
0.89 |
10.5 |
B |
501 c |
119 |
23.7 |
7.7 d |
2.45 |
30.9 |
|
C |
1134 a |
446 |
39.3 |
16.9 b |
11.17 |
66.2 |
|
|
D |
987 b |
354 |
35.2 |
12.3 c |
3.87 |
31.7 |
Table 3 Mean, SD and coefficient of variation (CV%) of two depths evaluated fewer than four locations for total & DTPA extractable Cd in different districts
*A, Al Zarqa; B, Faraskur; C, Kafr Saad; D, Damietta
** Means followed by the same letter within the row for Cd were not significant based on the least significant difference test at P < 0.05.
Data cleared that total Cd concentration in soils of Damietta governorate within a normal range in compared with the international standards and the permissible critical limits developed by the environmental authorities all over the world; from 0.06 to 1.10mg kg-1;7 from 3 to 6mg kg-1,14 and from 1.69mg kg-1 to 2.35mg kg-1.15
The districts average concentrations of surface soils, Cd are lower in both Faraskur district (519±126 µg kg-1) and El-Zarqa district (582±063 µg kg-1), while the highest values are found in both Damietta district (1012±433 µg kg-1) and Kafr Saad districts (1171±543 µ kg-1). The high values in Damietta and Kafr Saad regions may be attributed to the high rates of phosphorus fertilization application and irrigation with sewage effluent or mixed water. These findings are agreed with several research work48,49 and they reported that sewage sludge, soil organic matter and phosphorus fertilizers are important factors that influenced the enrichments of Cd in the topsoil.
Frequency distribution of total Cd in the studied soils shows that 26% of the studied soils contained values of total Cd less than 500µg kg-1 (Figure 5). These groups of soils contain normal values of total Cd comparing with the background levels of Cd in the world soil according to Pendias and Pendias.7 They found that the worldwide mean of total Cd was 0.53mg kg-1 in soils surface. While approximately 42% of the studied soils showed total Cd concentration range of 500 to 1000µg kg-1. Soils contain relatively high levels of total Cd (from 1000 to 2000µg kg-1) represented 33% out of the studied soils and is located mainly in Kafr Saad and Damietta districts.
Soil DTPA extractable Cd
The DTPA extractable Cd concentration varies from district to district and the Cd extractable ranges from 2.95 to 58µg kg-1 with an average of 16.41±13.83 µg kg-1 in the surface soils (Figure 6). While, the Cd extractable ranges from 1 to 37.38µg kg-1 with an average of 12.57±8.34µg kg-1 in the subsurface layers. These results agree with several studies in Egypt5,6,50 and they found the values of extractable Cd in Egyptian soils varies widely among the different soils, being from non-detected values to 60 µg kg-1 with an average of 18±2.0µg kg-1, Rashad et al.6 and Aboulroos et al.5 recorded 30 to 60µg kg-1 for DTPA extractable Cd form. Extractable Cd of the surface soil layers is slightly higher than the subsurface layers by 1.55µg kg-1 to 2.06µg kg-1 with an average of 1.31±0.45 fold. Thus, extractable Cd in the subsurface layers are close to its concentration in the surface layers. This may be due to the relatively higher amount of organic matter and the relatively low values of soil pH of the soil surface layers. Under such conditions the solubility of Cd in the surface soil layers and its downward movement to the soil subsurface layers increases. This agree with Wang et al.48 which reported that soil organic matter important is factors that influenced the enrichments of Cd in the soil.
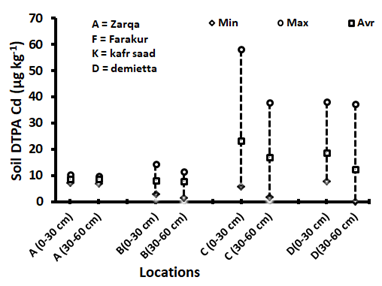
Figure 6 Minimum, maximum and average of soil DTPA extractable cadmium concentrations in the different districts.
The district average concentrations of surface soil Cd are lower in Faraskur district (7.94±2.51µg kg-1) and El-Zarqa district (8.62±1.17µg kg1), comparing in both Damietta district (18.57±11.13µg kg-1) and Kafr Saad district (23.06±17.23µg kg-1, Table 3). The high values in Damietta and Kafr Saad districts may be attributed to high rates of phosphorus fertilization and irrigation with sewage effluent or mixed water (Nile water + sewage effluent).
The frequency distribution of extractable Cd in the studied soils shows that 56% of the studied soils contained less than 10µg kg-1 of extractable Cd, while 39% of the studied soils contained from 10 to 40µg kg-1 extractable Cd (Figure 7). Soils contain relatively high levels of Cd from 40 to 80µg kg-1 represented 3% of the studied soils which mainly located in Kafr Saad and Damietta districts.
Extractable Cd increased linearly with increasing total soil Cd (r=0.90), and represent a small fraction of the total Cd accumulated in the soils, being in the range from 0.79 to 3.50% with an average 1.53±0.41% for entire studied soils (Figure 8). The highest percentages exist in Damietta and Kafr Saad districts may be attributed to the relatively higher organic matter contents and the lower in soil pH. There are no correlation between available Cd and the clay content of studied soils. A significant and positive correlation coefficient was found between soils extractable Cd and soil OM content (r=0.95), while significant negative correlation coefficient for CaCO3 content (r=-0.92) and pH (r=-0.94).
Mean of the total and DTPA extractable-Cd across four locations are presented in Table 3. The depth 0 to 30cm shows a significant variation (P<0.05) and the highest mean values over all locations for DAPT extractable. On the other hand, the total shows insignificant difference between the depths. Effect of locations on the total and DTPA extractable-Cd across the depths had a significant difference. Kafr Saad (location C), are observed the highest mean values for total and DTPA extractable-Cd. On the other hand, Faraskur (location B) are recorded the lowest mean values for total and DTPA extractable-Cd. Results show a significant effect for the interaction between depths and locations at the (P<0.05) for total and DTPA extractable-Cd. The locations show a significant variation in their means under depths (0 to 30 and 30 to 60cm) for total and DTPA extractable-Cd, the highest means under 0 to 30 and 30 to 60cm are achieved by Kafr Saad (location C) for total (1175 and 1134µg kg-1, respectively). On the other hand, Al Zarqa and Faraskur (location A and B) had the lowest mean values for both depths. Kafr Saad (location C) under 0-30 cm is observed the highest mean values for DTPA extractable-Cd (23.1µg kg-1). On the other hand, Al Zarqa and Faraskur (locations A & B) under both soil depth were recorded the lowest mean values for DTPA extractable-Cd (12.2µg kg-1).
Cadmium in plants
Cadmium in rice plant
The concentration of Cd in the rice straw of all districts ranges from 132 to 711µg kg-1 with an average of 374±156µg kg-1 (Figure 9). The Frequency distribution of Cd concentrations in the studied rice straw samples are 74% less than 400µg kg-1 Cd; 20% between 400µg kg-1 to 600µg kg-1 Cd and only 7% more than 600 µg kg-1 Cd (Figure 4). As an average, Kafr Saad district has the highest concentration of Cd (408±115µg kg-1) in rice straw followed by Damietta district (411±215µg kg-1). The lowest average Cd concentration (317±183µg kg-1) was found in rice straw collected from Faraskur district. These are the same trend of districts that contain the high or low DTPA extractable-Cd in the soils. A significant correlated with total soil Cd (r=0.89) and DTPA extractable Cd (r=0.84), respectively. This indicates that Cd taken up by roots is rapidly translocate to the straw. Whereas, in most plants species the transport of Cd onto shoot is usually in direct proportion to the external concentration.51–54
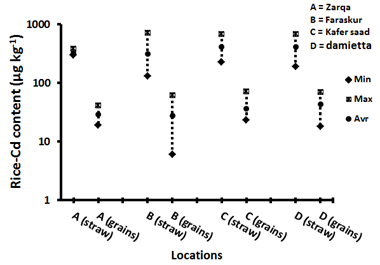
Figure 9 Minimum, maximum and average of rice parts cadmium concentrations in the different districts.
The concentrations of Cd in rice grains range from 6 to 71 with an average of 35±16µg kg-1 dry weight (DW; Figure 9). According to The threshold level proposed by the Codex Alimentarius Commission is 400µg kg-1 fresh weights (FW).55 Assuming that a rice grain contains approximately 23% water, the upper limit for Cd on a dry basis would be 520µg kg-1. Also, the threshold level proposed by the European Commission is 200µg kg-1 FW, which is 260µg kg-1 DW.56 However, one of the evaluated cultivars or accessions presented Cd concentrations in rice grains in excess of the abovementioned maximum allowable concentrations.
The frequency distribution demonstrates that 76% of the rice grains samples contain less than 40µg kg-1 Cd, 15% of the samples contain between 40µg kg-1 to 60µg kg-1 Cd and only 9% of the samples have Cd concentration more than 60µg kg-1 Cd (Figure 10). Damietta district maintains the highest concentration of Cd (43±22µg kg-1) in their rice grains, followed by Kafr Saad district (36±13µg kg-1). These are the same districts which exhibited the highest DTPA extractable-Cd in their soils. The least Cd in rice grains (27±17µg kg-1) and the least DTPA extractable-Cd recorded in soils are in Faraskur district and agrees with previous study57 and they found that Cd concentration in corn grains grown in normal non-polluted soils are less than 50µg kg-1. On the other hand, Dudas and Pawluk58 are also reported that Cd content of different cereal grains grown in different location in Canada is very similar in all types of grains and ranges from 40µg kg-1 to 60µg kg-1. The content of the rice grains in all the studied districts seems to be normal even those collected from soils with relatively high content of available Cd except the 9% fraction. A significant correlated Cd concentration are found in rice grains with total soil Cd (r=0.86) and DTPA extractable Cd (r=0.74), respectively. Figure 11 demonstrates that Cd concentration in straw and grains are highly correlated (r=0.98) and Cd content of rice grains represent between 4.6% to 10% with an average of 9.2±1.6% from that of rice straw content. The results agree with the findings of Sommer59 and Corguinha et al.,60 they found that Cd content of cereal grains from highly contaminated soils hardly exceeds one mg kg-1 (DW).

Figure 10 Frequency distribution of Cd concentrations in straw and grains of rice in the studied samples.
The Enrichment coefficient (EC) of Cd from soil to rice straw ranges from 0.39 to 0.59 with an average 0.46 whereas, transfer factor (TF) for Cd of rice grain ranges from 0.08 to 0.10 with an average 0.09 (Figure 12). This indicates that soil Cd level, organic matter content and pH are the major factors governing the Cd contents in rice straw and grins. This agrees with Bose and Bhattacharyya61 and Toth et al.,62 they refer the differences might be related to Cd-binding capacity, interactions between physicochemical parameters in soils.
Cadmium in wheat plant
The concentration of Cd in the wheat straw of all districts ranges from 94µg kg-1 to 345µg kg-1 with an average of 194±71µg kg-1 (Figure 13). Frequency distribution (Figure 7) of Cd concentration in wheat straw samples represents; 91% less than 400µg kg-1 Cd; 7% between 400µg kg-1 to 600µg kg-1 Cd and only 2% more than 600µg kg-1 Cd. As an average, Damietta district has the highest concentration of Cd (235±38µg kg-1) in rice straw, followed by Faraskur district (219±80µg kg-1). These are the same districts that contain the highest DTPA extractable-Cd in the soils. The lowest average Cd concentrations (152±66µg kg-1) are found in rice straw collected from Kafr Saad district. A significant correlation between Cd concentration in wheat straw with soil total Cd (r=0.90) and available Cd (r=0.92). This indicates that Cd up taken by roots is rapidly translocated to the straw. In this respect, Yashim et al.,52 Song et al.53 and Nurul et al.54 reported that in most plants species the transport of Cd onto shoot is usually in direct proportion to the external concentration.
The concentration of Cd in wheat grains is from non-detectable to 42 with an average of 18±13µg kg-1 (DW) (Figure 13). The maximum permitted Cd level in a wheat grain proposed by the Codex Alimentarius Commission is 200µg kg-1 FW.55 Assuming that a wheat grain contains approximately 16% water, the upper limit for Cd on a dry basis would be 240µg kg-1 DW. In this study, the results for Cd in wheat grains did not exceed this maximum allowable concentration. Figure 14 shows that 76% of the wheat grains samples contain are less than 40µg kg-1 Cd, 15% of the samples contain between 40µg kg-1 to 60µg kg-1 Cd and only 9% of the samples contain Cd concentration more than 60µg kg-1. Damietta district has the highest concentration of Cd (27µg kg-1) in their wheat grains followed by El-Zarqa district (20µg kg-1). These are the same locations having the highest available Cd in their soils. While, the least Cd in wheat grains (12mg kg-1) and least soil available Cd are shown by Faraskor district. Pietz et al.57 found that Cd concentration in corn grains grown in normal non-polluted soils was less than 0.05mg kg-1. Dudas and Pawluk58 reported that Cd content of different cereal grains grown in different location in Canada was very similar in all types of grains and ranged from 0.04mg kg-1 to 0.06mg kg-1. Cadmium concentration of wheat grains of all the studied fields is in the normal range even those collected from soils with relatively high content of available Cd except 9% of the samples. A significant correlation is found between soil total Cd (r=0.96) and available Cd (r=0.95) with Cd concentration in wheat grains.
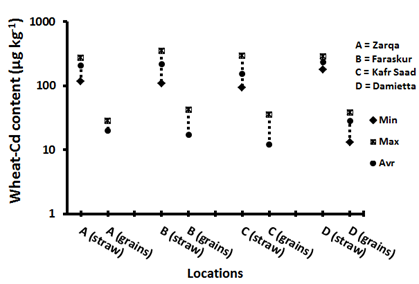
Figure 13 Minimum, maximum and average of wheat parts cadmium concentrations in the different districts.
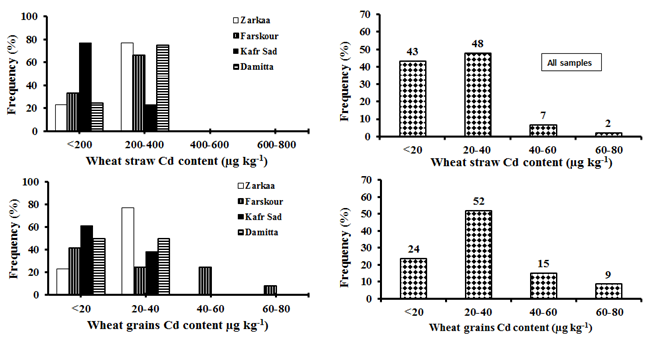
Figure 14 Frequency distribution of Cd concentrations in straw and grains of wheat in the studied samples.
The Cd content in wheat grains represents an average of 9.2% from that of wheat straw. Figure 15 shows that the two values of Cd in wheat straw and grains are highly correlated (r=0.95). This agrees with Sommer59 and Corguinha et al.60 who is found that the Cd content of cereal grains from highly contaminated soils hardly exceeds 1.0mg kg-1. Pendias and Pendias7 found that the background levels of Cd in cereal as well as in common feed plants (that are reported for various countries) are fairly low and sparingly similar. The grand mean for all cereal grains range from 0.013mg kg-1 to 0.220mg kg-1 (DW).
The Enrichment coefficient (EC) of Cd from soil to wheat straw (Figure 16) was ranged from 0.14 to 0.43 with an average 0.26 whereas, transfer factor (TF) for Cd of wheat grains ranges from 0.06 to 0.12 with an average 0.08.
The mean performance of straw, grain and grain/straw across two crops are showed a significant difference (P<0.05; Table 4). For straw, grain and straw/grain, it could be concluded from the obtained results that the rice genotype recorded the greatest values for straw, grain and straw/grain (375, 34.6 and 9.1), respectively. While in locations shows a significant difference for grain and straw/grain only, Damietta is recorded the highest values of grain and straw/grain (35.3 and 11.0). Result shows a significant effect for the interaction between crops and locations at the (P<0.05 that indicates the interaction between rice and both locations (Damietta and Kafr Saad) give the highest values of straw 411µg kg-1 and 408µg kg-1, respectively. Also the same crops and Damietta gave the highest values of grain (42.5µg kg-1). The interaction between wheat and location (locations give the highest values of grain/straw (11.7µg kg-1), followed by rice (10.4µg kg-1) in Damietta (location D).
Crops |
Location* |
Plant Cd concentrations mean** (µg kg-1) |
||||||||
Straw |
Grains |
Grains /Straw |
||||||||
Mean |
±SD |
CV% |
Mean |
±SD |
CV% |
% |
±SD |
CV% |
||
Wheat |
194 b |
71.2 |
36.8 |
17.8 b |
13.4 |
75.6 |
7.7 b |
4.9 |
63.1 |
|
Rice |
375 a |
155.6 |
41.5 |
34.6 a |
16.3 |
47.3 |
9.1 a |
1.5 |
16.5 |
|
A |
264 a |
69.3 |
26.2 |
24.5 b |
10.4 |
42.5 |
8.8 b |
3 |
33.8 |
|
B |
268 a |
147.6 |
55.1 |
22.2 b |
16.5 |
74.2 |
7.3 c |
3.6 |
49.6 |
|
C |
280 a |
158.8 |
56.6 |
24.4 b |
17.5 |
71.9 |
7.6 bc |
4 |
52.8 |
|
D |
323 a |
176.2 |
54.6 |
35.3 a |
18.1 |
51.4 |
11.7 a |
1.7 |
15.7 |
|
Wheat |
A |
209 c |
51.9 |
24.8 |
19.6cde |
9.8 |
50.1 |
9.3 b |
3.7 |
43.3 |
B |
219 c |
79.9 |
36.5 |
17.1ed |
14.9 |
87.1 |
7.8 c |
4.6 |
73.6 |
|
C |
152 c |
65.7 |
43.1 |
12.3 e |
12.9 |
105.2 |
8.1 c |
5.3 |
85.3 |
|
D |
235 bc |
37.8 |
16.1 |
28 bcd |
8.7 |
31.1 |
11.9 a |
2.2 |
18.9 |
|
Rice |
A |
320 ab |
25.8 |
8.1 |
29.4 bc |
8.8 |
30.1 |
9.8 b |
2.2 |
24.2 |
B |
317 ab |
182.9 |
57.7 |
27.4 bcd |
16.9 |
61.5 |
8.5 b |
1.9 |
22.4 |
|
C |
408 a |
114.5 |
28.1 |
36.5 ab |
12.5 |
34.3 |
8.8 b |
0.8 |
8.8 |
|
|
D |
411 a |
214.9 |
52.3 |
42.5 a |
22.1 |
52.1 |
10.4 ab |
0.7 |
6.5 |
Table 4 Mean, standard deviation and coefficient of variation (CV%) of two crops evaluated fewer than four locations for straw and grins Cd concentrations of the different districts
*A, Al Zarqa; B, Faraskur; C, Kafr Saad; D, Damietta
** Means followed by the same letter within the row for Cd were not significant based on the least significant difference test at P<0.05
Human health risk
It is clear that the studied locations not only increase Cd contents in the straw but also in the grains of rice and wheat. The high levels of Cd in both rice and wheat grains will decrease their demand for human consumption. Excessive Cd in the diet has been found to impair kidney function, disturb the metabolism of Ca and P and cause bone disease.63 The range of Cd concentration in rice grains ranges from 6µg kg-1 to 71µg kg-1 with an average of 34µg kg-1. Whereas, Cd content of wheat grains ranges from undetected to 42 with an average of 18µg kg-1. Thus, compared with the above mentioned limits the tolerable daily intake of Cd (1µg kg-1 day-1 FAO/WHO64 at maximum levels can be reached when a person consumes one kg of rice grains or 1.67kg of wheat grains per day. Therefore, the dietary intake of these grains results shows low level body accumulation of Cd and the detrimental impact becomes apparent only long time consumptions. Also, calculated Health Risk Index (HRI) rearrested value is less than 1 indicates Cd in rice and wheat grains have no potential human health risk now but may be in future will be happened.65–67
Damietta governorate shows that cadmium concentration in the soil is within the permissible critical limits, established by the European Union and WHO/FAO, and indicates the possibility of using such land for growing agriculture crops. Total and DTPA extractable Cd slightly accumulated in the soil surface layers than the subsurface layers. The concentration of DTPA-available Cd is much lower than the total Cd, but it is correlated with total Cd. However, concentration of Cd is higher in rice and wheat straw than the grains. On the other hand, rice straw and grain contain higher Cd concentration than wheat straw and grain. This may be due to genetic variability between the two species. Calculated Health Risk Index (HRI) rearrested value indicates that Cd in rice and wheat grains have no potential human health risk at the moment.
The authors would like to thank the Faculty of Agriculture, Cairo University and Atomic Energy Authority, Inshas, Egypt for their valuable assistance especially, during the samples preparation for cadmium analysis in soils, plants and water samples by Graphite Furnace Atomic Absorption Spectrophotometer.
All author listed here declare no conflict of interest exists.
None.

©2021 Badawy, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.