MOJ
eISSN: 2573-2919


Research Article Volume 4 Issue 3
1East Coast Environmental Research Institute (ESERI), Universiti Sultan Zainal Abidin, Gong Badak Campus, Malaysia
2Faculty of Industrial Design and Technology (FRIT), Universiti Sultan Zainal Abidin, Gong Badak Campus, Malaysia
Correspondence: Abdul Rahman Hassan, East Coast Environmental Research Institute (ESERI), Universiti Sultan Zainal Abidin, Gong Badak Campus, 21300, Kuala Nerus, Terengganu, Malaysia, Tel +609-6688069, Fax +609-6688707
Received: May 29, 2019 | Published: June 24, 2019
Citation: Mansoor ADKA, Hassan AR, Sulaiman NA, et al. Development of low pressure reverse osmosis membrane for desalination process. MOJ Eco Environ Sci. 2019;4(3):133-139. DOI: 10.15406/mojes.2019.04.00145
Water shortage has become a real problem at global level and therefore, new and innovative technologies were established to provide sustainable solutions to water crisis. One of the effective approaches to resolve the global challenges is introducing the membrane-based desalination. Reverse Osmosis (RO) is a pressure driven membrane process which becoming increasingly popular and widely used for water purification applications that require high salt rejection such as brackish and seawater desalination. In this study, the influence of Sodium dodecyl sulphate (SDS) surfactant in producing the finest membrane for desalination were investigated in terms of performance, morphological structure and molecular orientation. From a polymer blending of polysulfone (PSF)/N-Methyl-2-Pyrrolidone (NMP)/polyvinylpyrrolidone (PVP)/sodium dodecyl sulphate (SDS) were formulated for making of low pressure reverse osmosis (LPRO) membrane. In order to examine the influence of SDS surfactant, different concentration from 0 wt% to 3 wt% were employed for desalination application of 10,000ppm (brackish water) and 50 000ppm (seawater). Experimental data showed that the increasing of 0.5wt% in surfactant produced higher pure water permeation (PWP) and flux. At 2.5wt% of SDS, the LPRO membranes showed the highest PWP of about 44.8L/m2h and brackish water flux at 45.58L/m2h. Meanwhile, at 3.0wt%, the highest flux of seawater at 39.37L/m2h was obtained. Moreover, the optimized LPRO (2wt% of SDS) membrane performed high rejection ratio of 90.9% for brackish water and 90.4% for seawater concentration of 10,000ppm and 50,000ppm, respectively. Therefore, the findings revealed that the fabricated LPRO membrane having a good potential to be used as eco-efficient desalination process of brackish water and seawater technology.
Keywords: low pressure reverse osmosis, surfactant, desalination, morphologies, molecular orientation
PSF, polysulfone; NMP, N-Methyl-2-Pyrrolidone; PVP, polyvinylpyrrolidone; SDS, sodium dodecyl sulphate; (SDS); PWP, pure water permeation; LPRO, low pressure reverse osmosis; RO, reverse osmosis; SEM, scanning electron microscope; FTIR, fourier-transform infrared; A, effective membrane area (m2); Cf, concentration of feed (µS/cm); Cp, concentration of permeate (µS/cm); Jv, permeate flux (L/m2h); ppm, part per million; R, rejection; t, time (h); V, volume of permeate solution collected (L).
Over the past decades, there are many countries in the world suffering from a shortage of natural fresh water. The rise in global population rates together with the expansion of industrial and agricultural activities have led to a dramatic increase in requirement of fresh water.1 Sandia defined that fresh water is containing less than 1000mg/L of salts or total dissolved solids (TDS).2 For above 1000mg/L, properties such as taste, color, corrosion propensity, and odor can be adversely affected. Available fresh-water resources from rivers and groundwater are presently limited and are being increasingly depleted at an alarming rate in many places. Due to population growth and increasing demand for water, new methods to create clean water have to be found. Conventional sources for fresh water such as rivers, lakes, and groundwater are overused or misused.
Akili and co-workers claimed that, seawater is unsuitable for human consumption and for industrial and agricultural uses.3 Membrane-based seawater desalination and wastewater reuse are widely considered as promising solutions to augment water supply and alleviate water scarcity.4,5 Now membrane processes are an efficient and reliable way to treat water and they have become better functioning and more cost effective than they were a few years ago. Reverse osmosis (RO) is one of established process and the leading technology for the desalination process of saline water which purify water by separating the dissolved solids from feed stream resulting in permeate and reject stream for a wide range of applications in domestic as well as industrial applications.6–8
Polymeric reverse osmosis (RO) membranes have dominated commercial applications for desalination due to their low-cost fabrication, ease of handling and improved performance in selectivity and permeability. Nowadays membrane applications spread over various industries including medical sector.9 For industry, low pressure reverse osmosis (lower than 100 bar of pressure required) is suggested to be used as an alternative as the lower pressure operation is very attractive in reducing capital and operation cost. It also can provide an easier method for the system maintenance and then achieve energy saving. Murthy and Choudhari10 studied the paper on the treatment of distillery spent wash where ultrafiltration (UF) and RO membranes on pilot plant uses thin film composite for purification of the wastewater for removal of colour and the contaminants. The obtained result indicates the suitability of RO for reducing freshwater consumption by recycling water which will minimize the waste disposal costs and reduction in regulatory pressure.
The desalination performance of RO membrane depends largely on the membrane material and the membrane structure. An industrially useful RO membrane must exhibit several characteristics such as high water flux, high salt rejection, mechanical stability, tolerance to temperature variation, resistance to fouling, and low cost. So far, a number of polymer materials have been used to make RO membranes.11 In this study, PSF had been used to produce LPRO membrane.
Polysulfone is a polymer widely used as a membrane material for liquid separation processes such as ultrafiltration and reverse osmosis. It consist both aliphatic and aromatic polymers that widely used as membrane since PSF has good resistant to high temperatures, good mechanical and chemically stable.12 These criteria make PSF the best membrane materials for separation process. The good solubility allows extensive uses of PSF membranes, with special emphasis on phase inversion by immersion precipitation.13 However, PSF exhibit two main disadvantages which are the hydrophobic character and the low resistances to UV radiation. Thus, suitable materials need to be introduced in the casting solution in order to prepare membranes with excellent penetration properties.
The modification of LPRO membranes can be improved by the addition of small amount of additives into the casting solution as it can control the membrane formation. Various inorganic (such as LiCl) and high molecular weight organic such as polyvinyl pyrrolidone (PVP) or polyethylene glycol (PEG) additives to casting solution are often used.14 PVP and PEG are the most important polymeric additives used in study and evaluate the membranes performances. This is because they are miscible with the most of the membrane materials and are soluble in both aqueous and many organic media.15 Ochoa and co-author confirmed that the addition of PVP to the casting solution increases the UF-PES membrane permeability without significant changes in selectivity.16 Marchese and team reported that an increment in the pore density, a decrease of the effective thickness of the dense layer due to macrovoids in the support layer and an increment in the hydrophilicity of the surfaces on the membrane and inside the pores are the reasons behind the increase of membrane permeability when PVP is added.17
Furthermore, one of the effective strategies to produce membrane with high performance in separation process is introducing surfactant as a new material in the casting solution. Since surfactant as additive were found to affect the performance and morphological structures of membrane, the potential of the surfactant towards production of newly LPRO membrane was studied. In this study, the effects of anionic surfactant (SDS) on the performance, morphology and orientation of the newly LPRO membrane was investigated. In context of application in water desalination, at different range of salt concentration, the effectiveness of LPRO was studied. Moreover, this study also provided a length of study towards the determination the best technical specification of asymmetric membrane to be used for desalination application. Ultimately, this research shows potential in generating an important knowledge on the roles of surfactant and major effects which is truly beneficial towards the advancement in membrane fabrication principle for the production of new membrane for different application in the future.
Materials
The starting materials, Polysulfone (PSF (Udel-P1 700); from Solvay), N-methyl-2-pyrrolidone (NMP; from Merck), Polyvinylpyrrolidone (PVP (K29-32); from Across Organics) and Sodium dodecyl sulphate (SDS; supplied by Fisher Scientific UK) were used for fabrication of LPRO membranes. Ethanol and n-hexane were used for pre-treatment step. Sodium chloride (NaCl; from Merck) also included in material selection as to study the performance of salt rejection.
Preparation of LPRO membranes
In this study, Table 1 shows the dope formulation solution of the PSF dissolved in NMP was prepared using PVP as additive and different surfactants by stirring for 8h at room temperature. The homogenous dope solution of membranes were cast on a glass plate using casting knife and having the thickness about 150µm. Subsequently, the membranes were immersed into water for immersion precipitation process and remained for 24h in order to ensure complete phase separation. For solvent-exchange process, membranes were immersed in ethanol for 24h and n-hexane for 3h. Then, the membranes were ready to be used after drying process for 24h.
NMP |
PSF |
PVP |
SDS |
(wt%) |
(wt%) |
(wt%) |
(wt%) |
76 |
21 |
3 |
0 |
75 |
21 |
3 |
1 |
74.5 |
21 |
3 |
1.5 |
74 |
21 |
3 |
2 |
73.5 |
21 |
3 |
2.5 |
73 |
21 |
3 |
3 |
Table 1 The dope formulation
Note: NMP, N-methyl-2-pyrrolidone; PSF, polysulfone; PVP, polyvinylpyrrolidone; SDS, sodium dodecyl sulphate
Membrane performance evaluation
The reverse osmosis (RO) filtration setup was used to investigate the desalination performances of seawater in terms of pure water permeation (PWP), flux and salt rejection. The membrane area for the LPRO membrane system was 1.38x10-3 m2 with pressure of 5 bars. The testing was begin using deionized water pressured at 5 bars for compaction at least 30min. then, the test were carried out with aqueous solution containing 10,000ppm and 50,000ppm which presenting the brackish water and seawater, respectively. The water permeability of the prepared membranes can be described based on equation (1):
(1)
Where Q is the volume permeation (liter), A is the effective area of the membrane for permeation (m2) and t is the time interval (hour). In addition, the flux and salt rejection for the RO system were calculated using the Equations (2) and (3), respectively.
(2)
Where Jv is the permeate flux of solution, V is the volume of permeate solution collected (liter), A is the effective area of membrane (m2) and t is the time (hour).
(3)
Where R is rejection of salt in percentage and andare the concentration of permeate and feed water, respectively. The salt concentration was investigated by measuring the electrical conductivity of the salt solution using a conductivity meter (SensION EC5, HACH Instrument).
Morphology evaluation
Analytical scanning electron microscopes (SEM, JSM 6360LA) was used to observed the morphology of the LPRO membranes. The cross-sectional views were examined as the membranes were immersed and fractured in liquid nitrogen, then sputter-coated with gold for producing electric conductivity.
Molecular orientation evaluation
The orientation of molecule in LPRO membrane samples were examined by transmission Variance 3100 Fourier-transform infrared (FTIR) Excalibur series. The analysis was done to study the functional group of the membrane. The sample was mounted into the sample holder with ‘skin layer’ facing the infrared (IR) beam. The spectra were recorded with cumulating 32 scans in total within the wave number of 4000-400cm-1. The analysis was performed at 4cm-1 of resolution.
Performance of LPRO membranes
Membrane formulation greatly influenced the performance of RO membrane. Composition of the surfactant in membrane solution will affect the performance of the resultant membrane. The SDS concentration was varies from 1wt% to 3wt%. The pure water permeability of the membranes was investigated using distilled water under 5 bars operating pressure. From the result obtained in Figure 1, at 1wt% of SDS, the fabricated membrane produced the permeability about 15.11L/m2h. By increasing 0.5 wt% of surfactant concentration in the casting solution, the PWP increased and reached the maximum value of about 44.8L/m2h at 2.5wt% of SDS. At 3wt%, PWP showed an inverse trend of permeability with reduction of about 57.4%. Addition of high amount of surfactant concentration leads to higher viscosity of solution which inhibits the penetration of non-solvent and weaken macrovoid formation and therefore, resulting in decreasing of membrane porosity and permeability.18,19
The fabricated membranes (PSF/NMP/PVP/SDS) were then evaluated for the performance. The influence of anionic surfactant on brackish water flux and rejections is depicted in Figure 2. It was clearly showed that membrane with 2.5wt% of SDS, shows the highest value of flux of about 45.58L/m2h among all of other membranes. The addition of small amount of SDS surfactant promotes higher porosity of membrane sub-layer which determined higher water flux.20,21 Moreover, the fabricated membranes shows the improvement of salt rejection from 85.7% (without SDS) up to 88% in the presence of 1wt% of surfactant. The increasing concentration of surfactant up to 2wt% produced the maximum salt rejection value of about 90.9%.
The effect of SDS concentration on flux and rejection of seawater are shown in Figure 3. It can be seen from that the flux of all fabricated membranes were increased gradually and achieved the highest value of about 39.37L/m2h at 3wt% of SDS. This is because the increment of SDS surfactant makes the membranes structure more porous in terms of formation of larger macrovoids which leads to higher volume of water flux produced.18 Same study by Rahimpour and his group reported that the small addition of surfactants (SDS, CTAB and Triton X-100) into casting solution increases the porosity of the membrane support layer which enhances pure water flux and milk concentration.22 As presented in Figure 3, the salt rejection for 50 000 ppm generally increased as the polymer concentration increased. The maximum value of the rejection is about 90.4% at 2wt% of SDS. Nevertheless, the rejection started to decrease to 88.74% and 86.92% as the concentration of the surfactant increased to 2.5wt% and 3.0wt%, respectively. This phenomenon might due to the anionic surfactant at higher concentrations interfered with the interfacial polymerization which deteriorating the structure of the fabricated membranes.
From Figure 2 and Figure 3, 2 wt% is the optimum concentration of SDS for the membranes as it depicted the maximum rejection of salt in brackish and seawater analysis. The optimal performance of the membranes at this point might be due to the increasing of membrane porosity and lower skin layer thickness, thus, resulting in the increment of permeability rate.18
Morphological study of LPRO membranes
The surface morphology of the polysulfone membranes with different concentration of SDS was shown in Figure 4 (a)-(f). The SEM images indicated that the addition of surfactant causes an increase in the formation of micro and macrovoids, depending on the use of surfactant concentration. In this study, all the fabricated membranes exhibit asymmetric structures with a combination of two layers, which are active layer and supporting layer that have significant role in membrane performance.
SEM cross-sectional images of membrane without surfactant were shown in Figure 4(a). It revealed a skin layer that was well-developed and supported by a porous support layer with large finger-like structure and microvoids. In Figure 4(b), at 1wt% of surfactant, the fabricated membrane formed macrovoids and finger-like structure were improved compared with the membrane without surfactant. This might be due to the addition of surfactant in the dope formulation as the molecule distribute freely in solvent and because of high polymer concentration which was 21wt%, the others could form micelle-like polymer-surfactant complex.23,24 Fadilah and Hassan25 stated that composition of surfactant in membrane solution will affect the performance of the membrane as it plays a significant role in improving macrovoid structure and thickness.25 Usually, the large finger-like structure and macrovoid are formed when the coagulation process is faster.26
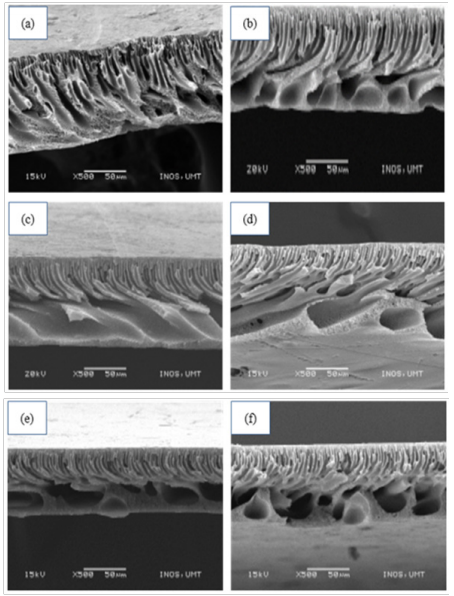
Figure 4 SEM cross-sectional images of LPRO membranes prepared with different concentration of SDS: (a) 0wt% (b) 1wt% (c) 1.5wt% (d) 2wt% (e) 2.5wt% (f) 3wt%.
Meanwhile, Figure 4(c) shows that the presence of 1.5wt% of SDS surfactant produced a thicker top layer and suppressed the macrovoids. This phenomenon might related to the finding of Saedi and co-workers which mentioned that molecules can form free micelles at higher concentration of SDS and they preferred to form free micelles rather than to form a polymer-surfactant complex, so the formation of micelle-like polymer-surfactant complex suppresses.18 In particular, membrane in Figure 4(d) produced larger size of macrovoids as compared to other membranes, hence, improved the permeability performance which might be contributed by larger pore size of the membrane.27 This optimal performance membrane exhibits characteristics morphology of asymmetric membrane which consisted of a dense top layer and porous sub-layer.28 Zainal and team mentioned that SDS makes the membrane more porous as it formed larger macrovoids.29
Figure 4(e) and Figure 4(f) demonstrated of membranes with surfactant concentration at 2.5wt% and 3wt%, respectively. The morphology analysis revealed that denser top layer and fully develop finger-like structure were formed. Furthermore, the result shows that, there were an increasing number of pores produced in the membranes as compared to the membrane of lower surfactant concentration. The spongy structure of the membranes gradually becomes compacted. It reduced the porosity of membrane support layer and resulted in lower PWP and salt performance.
Molecular orientation study of LPRO membranes
Figure 5 and Figure 6 shows the FTIR spectrum of membrane without surfactant and membrane with optimal performance of desalination at 2wt% of surfactant, respectively. FTIR plays a decisive role in order to study the intermolecular interaction between the molecules in the membrane.
In Figure 5, the spectrum of membrane without surfactant shows the transmittance band observed near 3412 cm-1 which assigned to O-H stretching vibration of PSF polymer. The presence of aromatic C=C, C=O and S=O bonds were belong to PSF polymer and interpreted at 1585cm-1, 1243 cm-1 and 1151 cm-1, respectively. These results were agree with the finding of Mehta and co-author and study by Karimi and co-workers.30,31 In addition, the transmittance band observed at 1490 cm-1 can be explained owing to the C-N bond stretching vibration which proved that the presence of PVP as additive in the membrane. This transmittance band value can be related with the previous study which mentioned that the C-N stretch value is within 1112-1595 cm-1.32 The similarity of infrared spectrum confirmed that PSF membranes display about same activity even after the existence of additive and surfactant.
FTIR spectrum for optimal performance membrane at 2 wt% of surfactant was shown in Figure 6. The FTIR spectrum in this figure does not showed significant changes compared to Figure 5 due to similar chemical solutions used as the base materials in membrane making. However, no major peak observed for SDS molecule as displayed. Yet, a C-H3 stretching at 2864 cm-1 confirmed the existence of SDS surfactant. This peak in line with the finding of Viana and co-authors which discover the stretching of C-H3 group from SDS at 2873 cm-1.33
High selective LPRO membrane based surfactant for desalination was successfully developed. The effect of varying the concentration of SDS surfactant during the membrane formation was studied. Experimental data indicated that the small amount of surfactant (at 2.5wt% of SDS in the polymeric solutions significantly improved the membrane permeability up to 44.8L/m2h and 45.58L/m2h for brackish water flux. Rejection data revealed that at the optimal SDS concentration (2wt%), the membrane achieved highest rejection of salt about 90.9% and 90.4% for of brackish and seawater, respectively. The fabricated membranes exhibit of asymmetrical structures with the formation of fine finger-like structure, macrovoids and number of pores on the active layer and supporting layer which enhanced the performance of membranes. Furthermore, molecular study of LPRO membrane proven that the addition of surfactant in the polymeric membrane solution provided better molecular orientation which reflected towards better performances.
None.
The authors declared there is no conflict of interest.

©2019 Mansoor, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.