MOJ
eISSN: 2573-2919


Research Article Volume 6 Issue 1
Department of Chemistry, Injibara University, P.O. Box 40, Injibara, Ethiopia
Correspondence: Melaku Metro, Department of Chemistry, Injibara University, P.O. Box 40, Injibara, Ethiopia
Received: February 03, 2021 | Published: February 26, 2021
Citation: Adamu F, Metto M, Kassie B. Determination of heavy metals in soil used for potato cultivation by atomic absorption spectroscopy in awi Zone, Amhara Region, Ethiopia. MOJ Eco Environ Sci. 2021;6(1):28-33. DOI: 10.15406/mojes.2021.06.00210
The study evaluates heavy metals in soil used for Potato cultivation by Atomic Absorption Spectroscopy in awi Zone, Amhara Region, Ethiopia. In this study, 75 soil samples from three agricultural areas, namely: Guaguasa Shikudad, Fagita Lecoma and Banja districts were collected and analyzed for Lead (Pb), Cadmium (Cd), Nickel (Ni), Copper (Cu) and Zinc (Zn) using Atomic Absorption Spectroscopy. The results obtained revealed that the mean heavy metal concentrations for Pb, Cd, Ni, Cu, and Zn were 75.33–77.00mg/kg, 17.11–18.76mg/kg, 60.26-101.78mg/kg, 125.17-383.39mg/kg and 244.20—287.87mg/kg respectively. In general, the level of metals in soil collected from the sampling sites are found to be decreased in the order of: Cu>Zn>Ni>Pb>Cd. Soil analysis showed that the concentration of Cu, Cd and Ni higher than permissible levels there WHO/FAO thresholds for agricultural soils the soil is polluted by toxic heavy metals (Cu, Cd and Ni). Therefore, the soils studied were harmful for the cultivation of potatoes and other agricultural purposes.
Keywords: potato cultivation, atomic absorption spectroscopy, heavy metals, geological processes
Soil is an unconsolidated mineral and organic material found on the immediate earth surface that serves as a natural medium for plant growth and other developmental activities.1 Heavy metals occur naturally in soils, which are formed by geological processes, such as alteration and erosion of the geological underground materials.2,3 Ethiopia is among the top potato producers in Africa, with 70 % of its arable land in the high altitude areas being suitable for potato production.4 In 2013, Ethiopia stood in the 10th position of African countries in production estimating that production has increased from 280,000 tons in 1993 to around 775,503 tons in 2013.5 There is also a risk of contamination of soils with excess heavy metals because of atmospheric deposition, the use of pesticides, and fertilizers that contain considerable amounts of metals.6 The regulated levels of heavy metals that occur naturally in the soil environment from the pedogenetic processes of weathering of parent materials at levels that are regarded as trace (<1000mg/kg) and rarely toxic.7 However, information about heavy metal contents in soils in the study areas is scarce because no one has been done on the soil in the area.
Because of the mentioned effects of heavy metals on potato growth, the scarcity of the effect of the heavy metal on the soil for potato cultivation and their regulations limited by concerning bodies, it is necessary to determine the number of heavy metals in soils for potato cultivation by using spectroscopic methods, hence, monitoring their concentration in soils may prevent soil erosion and control many problems of plant and crop cultivation and growth like a potato.
Various methods including; AAS, AES, UV-Vis, etc. have been employed for the determination of heavy metal concentrations in soil samples. Atomic absorption spectroscopy (AAS) has for many years been one of the chemist’s favorite analytical tools for quantitatively measuring very small amounts (trace amounts) of metallic elements in samples as varied as alloys, rocks and soils, foods and drinks, surface waters, biological fluids, and reagent chemicals. Although the sensitivity of this technique varies from metallic element to metallic element as well as with the method of excitation (flame vs. graphite furnace, for example), this technique is generally capable of accurately measuring a metallic element’s concentration in a solution in the 1 to 100ppm range. Because of its ability to measure the concentration of over 60 of the metallic elements at extremely low concentrations, AAS has many analytical applications including impurity analysis of substances, forensic analysis, biomedical tissue, environmental samples and chemical research.8
Therefore, This study aims to determine the heavy metal content of the soil used for potato cultivation in Awi Zone in Amhara Regional State, Ethiopia. lamp background correction and hollow cathode lamps used for the analysis of the heavy metals (Ni, Cu, Zn, Cd, and Pb). Electronic analytical balance (AA-200DS, Deriver Instrument Company, Germany) was used for weighing the samples.
Chemicals and reagents: Aqua regia (prepared from 3:1 ratio of 35–38% HCl and 65–68% HNO3) and 30% H2O2 (Uni-Chem®Chemical reagent, India) were used for sample digestion. HClO4 (99%, KIRAN LIGHI Laboratories, India) was used as a matrix for the method blank. 1000ppm stock standard solutions of the heavy metals of Zn, Cu, Ni, Cd, and Pb (India) were used to prepare the calibration standard and spiking standard solutions. Double distilled water was used throughout the study. The glassware and polyethylene containers used for analysis were washed with tap water, then soaked overnight in 10% (v/v) HNO3 solution and rinsed several times with double distilled water to eliminate absorbance due to the detergent.
The dried soil samples were grounded with a porcelain mortar and pestle, passed through 0.5mm sieves, and then kept in clean polythene bags for further analysis.
Sample digestion: Wet digestion method was used for the digestion of the soil samples. 0.5g of each of the air-dried, ground, and sieved soil samples were accurately weighed into a 300ml calibrated digestion tube. 3ml concentrated HNO3 (in 2.3. Sample collection and pretreatment temperature: A total of 75 soil samples were collected from February to March 2018 from three different agricultural areas that are used for the cultivation of potatoes using soil samplers from three woredas, i.e., 25 samples from each woredas (randomly five kebeles were selected from each woreda and each kebele total of five samples were collected) total sample is 3*5*5=75, the soil samples were collected from top soil at the depths of 0–20cm using a soil auger. From each of the three agricultural sites, five subsites were taken for random sampling, and five soil samples were randomly collected from each of the five subsites in every three agricultural areas and pooled together to obtain a composite sample. The samples were air dried for one week at Injibara University Chemistry laboratory.
The fume hood), and swirled carefully and then the tubes were placed in a rack. Then the tube racks were placed in the block digester. Then after the glass funnels were placed in the neck of the tubes. The temperature was slowly increased setting to about 145˚C for one hour. After 4 ml of HClO4 was added and heated to 240˚C for 1 hour. The tube rack was lifted out from the block digester aqua and the rack holder was placed carefully and then test allowed the tubes to cool at room temperature.
The solution was filtered through Whatman No. 42 filter paper and brought to a volume of 50ml with double distilled water. Each sample was digested in replicates of five and transferred to acid-washed stoppered glass bottles, labeled and kept for metal analysis.
Method validation and quality control: To validate the analytical method, the method validation parameters such as ILD, LOD, LOQ, precision, and accuracy studies were carried out.9
Instrumental detection limit and limit of detection: IDL is the smallest signal background noise that an instrument can detect reliably.9 In this study, the IDL for each metal was determined as:
Where Sbl is the standard deviation of the calibration of the blank
The percentage recoveries of the analyte were calculated to evaluate the accuracy of LOD is the minimum concentration of analytes that can be detected but not necessarily quantified with an acceptable uncertainty. LOD for each metal was determined9:
Where Sbl is the standard deviation of the method blank
Precision and accuracy: Precision and accuracy of the results were assessed by determining the recovery and repeatability of the analysis of matrix spike, matrix spike duplicate, and laboratory control samples. For doing so, each sample was spiked in replicates of three at near mid-range calibration concentrations. The spiked samples were digested and analyzed following the same analytical procedure as the soil samples.
Precision was expressed as RSD of replicate results. The relative standard deviations of the samples were obtained as:
The analytical procedure. Recovery was then calculated as.9
Calibration blank and method blank: A calibration blank (2% HNO3) was prepared to determine the background signal and establish the baseline of the instrument. For the method, blank, 0.5g HClO4 was added as a matrix and prepared using the same digestion procedure described for the soil sample but with no added sample.
Determination of heavy metals: The instrument will calibrate using a calibration blank and five series of working standard solutions of each metal will be analyzed. The digested samples determined for the concentrations of heavy metals (Ni, Zn,Cu, Cd, and Pb) using FAAS, Model: AA-320N, Shanghai, China) at Amhara Design and supervision works Agency on Bahir Dar by following the operating conditions in Table 1.
|
Element |
Wavelength (nm) |
Silt width (nm) |
Current (mA) |
Flame type |
Energy |
Instrument model |
|
Cu |
324.7 |
1.2 |
2 |
air acetylene |
257 |
NovAA 400P AAS |
|
Zn |
213.9 |
0.5 |
2 |
air acetylene |
439 |
|
|
Pb |
283.3 |
0.2 |
2 |
air acetylene |
358 |
|
|
Cd |
228 |
1.2 |
2 |
air acetylene |
387 |
|
|
Ni |
232 |
0.2 |
3 |
air acetylene |
453 |
|
Table 1 Instrument operating conditions in soil samples by FAAS
Regression analysis and detection limits: As can be seen from Table 2, the calibration curves for the metals showed good linearity with coefficients of determination (r2) ranging between 0.9990 and 0.9997, which were greater than the acceptable limit (0.998) for the linearity of the regression line.10 This showed that there is a good correlation between concentration and absorbance indicating good calibration of the instrument. The IDL ranged from 0.00039 to 0.0195mg/L for all metals which were below the MDL, indicating the good sensitivity of the measuring instrument for analysis.
|
Metal |
IDL(mg/l) |
LOD(mg/kg) |
LOQ(mg/kg) |
Regression coefficient, r2 |
|
Cu |
0.00195 |
0.255 |
0.85 |
0.9997 |
|
Zn |
0.00105 |
0.186 |
0.62 |
0.9997 |
|
Cd |
0.00063 |
0.135 |
0.45 |
0.999 |
|
Pb |
0.00123 |
0.168 |
0.56 |
0.9993 |
|
Ni |
0.00039 |
0.072 |
0.24 |
0.9994 |
Table 2 Linear regression equations, coefficient of determination, IDL and LOQ
The MDL value lied in the range of 0.072–0.255mg kg-1, This is an indication of the sensitivity of the technique. The method quantification limit (LOQ) value lied in between 0.24 and 0.85 mg kg-1. The result shows that both the MDL and LOQ values were greater than the IDL; hence, the results of the analysis could be reliable.
Accuracy and precision: As can be seen from Table 3, the mean percent recoveries for the studied metals in the matrix spike sample ranged between 96.4 and 101.80%. All recovery values were within the acceptable range of 80–120% for metal analysis.11 The precision of the method was expressed as RSD of the five replicate readings. The RSD values obtained for standard soil matrix spike samples ranged from 1.05 to 3.162% (Table 2), which was under the required control limits ≤15%.12 These indicate that the proposed method was precise and accurate.
|
Metal |
Conc. In sample (mg/kg) |
Amount added (g/l) |
Conc. in spike sample(g/l) |
Recovery (%) |
RSD (%) |
|
|
Cu |
379.62 ± 8.02 |
100 |
477.33 ± 8.75 |
97.7 ± 1.174 |
1.2 |
|
|
Zn |
266.86 ± 9.11 |
100 |
365.20 ± 10.70 |
98.3 ± 3.108 |
3.16 |
|
|
Cd |
18.43 ± 0.57 |
100 |
120.26 ± 1.10 |
101.8 ±1.069 |
1.05 |
|
|
Pb |
89.13 ± 4.02 |
100 |
188.46 ± 4.60 |
99.3 ± 2.24 |
2.25 |
|
|
Ni |
96.86 ±1.47 |
100 |
193.30 ±2.72 |
96.4 ± 1.26 |
1.30 |
Table 3 Recovery and precision test results of soil samples with matrix spike
The concentration of heavy metals in soil samples: The mean concentrations of heavy metals in the soil samples are shown in Table 4 and Figure 1. Results are expressed as mean±standard deviation of five replicate analyses. The Copper (Cu) was highest among the heavy metals analyzed from the sample sites and the evels obtained were found to be 383.39mg/kg in Guagusa Shikudad soil sample, 223.81mg/kg in Banja soil sample and 125.17mg/kg in Fagita Lecoma soil samples. The lowest copper (Figure 1) accumulation was found in Fagita Lecoma soil sample and the highest concentration was found in the Guagusa Shikudad soil sample that 383.39mg/kg. From the experimental data, the revealed data indicated that the concentrations of copper in the three agricultural sites are higher than the regulated levels. The regulated level of copper in the soil samples was 100mg/kg according to WHO/FAO and 140mg/kg according to EU set.12
|
Metals |
Agricultural sites |
Max. Safe limits in soil (mg/kg) |
||
|
Guagusa Sh. |
Fagita lecoma |
Banja |
||
|
Cu |
383.39 ± 14.29 |
223.81 ± 15.98 |
125.17 ± 8.25 |
100, 140ab |
|
Zn |
268.34 ±10.06 |
244.20 ± 12.02 |
287.87 ± 5.59 |
300ab |
|
Cd |
18.76 ± 0.86 |
17.99 ± 0.67 |
17.11 ± 1.09 |
3c |
|
Pb |
77.00 ± 4.55 |
75.33 ± 2.94 |
76.10 ± 3.66 |
100c |
|
Ni |
96.02 ± 1.19 |
60.26 ± 3.61 |
101.78 ± 6.83 |
50, 75a b |
Table 4 Mean concentrations of metals (mean ± SD, n =3, mg/kg dry weight) in soil samples
The concentrations of Zn metal in the selected agricultural sites ranges from 244.20mg/kg to 287.87mg/kg. From the revealed concentrations of Zn, the highest quantity of a was obtained from Banja soil sample and the lowest of that was found from Fagita Lecoma soil samples. The concentrations of Zn in all three agricultural soil samples collected from the above sites were lower than that regulated by the WHO/FAO and EU. The maximum regulated limits for Zn in agricultural soils were 300 mg/kg according to WHO/FAO and EU, indicating that the levels of Zn in the agricultural places were safe for the cultivation of potato. The high level of Zn in these agricultural sites could probably be due to the widely used vegetable farming activities in these areas and the high usage of various types of pesticides and fertilizers.
The results of Cadmium (Cd) concentration were found to be 18.76mg/kg in the soil samples collected from Guagusa Shikudad agricultural sites, 17.99mg/kg in the soil samples collected from Fagita Lecoma agricultural sites and 17.11mg/kg in the soil samples collected from the Banja agricultural districts. The data reveals the highest content of Cd was found in the soil samples collected from Guagusa Shikudad agricultural site, which was 18.76 mg /kg and the lowest concentration of cadmium was found from the soil samples collected from Banja agricultural soil. From the experimental data, the revealed data indicated that the concentrations of cadmium in all three agricultural sites have shown the high accumulation of cadmium and there is a direct impact on the cultivation of potato as shown in Figure 2. This is because that the levels of cadmium in soil samples collected from the listed sites were higher than the normal regulated levels by WHO/FAO and EU. The regulated level of copper in soil samples was 3mg/kg according to WHO/FAO and EU set by13,14 as shown in Table 4. The metal-bearing solids at contaminated sites can be originated from a wide variety of anthropogenic sources in the form of disposal of high metal wastes in improperly protected landfills, land application of fertilizer, animal manures, biosolids (sewage sludge), compost, pesticides, coal combustion residues, and atmospheric deposition.15
The concentrations of lead (Pb) in the selected samples were found to be 77gm/kg from Guagusa Shikudad soil sample, 75.33mg/kg from Fagita Lecoma soil samples and 76.10mg/kg from soil samples that were collected from Banja agricultural site. In comparing the contents of metallic lead concentration in all three agricultural soil samples, the soils that were collected from Banja agricultural sites had the highest accumulation and the soils that were collected from the Guagusa, Shikudad revealed the lowest accumulation of lead as in Figure 3,4 and Table 4. From the experimental data, the concentration of lead in agricultural sites used for potato cultivation is not highly accumulated and it does not affect the growth of potatoes in Banja, Fagita, Lecoma, and Guagusa Shikudad districts.
The concentrations of Nickel (Ni) in the selected soil samples were found to be 96.02mg/kg in the soil samples that were collected from Guagusa Shikudad agricultural district, 60.26mg/kg in the soil samples that were collected from Fagita Lecoma agricultural district and 101.78mg/kg in the soil samples that were collected from Banja agricultural districts as shown in Table 4. From the result data, it can be seen that the concentration of Ni metal in the soil needed for potato cultivation selected from three different sites had considerable variations. The soil samples that were collected from Banja agricultural site have the highest concentration of metal and those collected from Fagita Lecoma agricultural sites have the lowest accumulation of Nickel that is 60.26mg/kg. it can also be seen that the soil samples that were collected from Banja areas contain high Nickel deposits even it is above the permissible levels regulated by the concerned bodies. The data (Table 4) revealed that the concentrations of nickel in Guagusa, Shikudad, and Banja soil samples were greatly higher than the permissible levels that were regulated by WHO and FAO jointly as well as the European Union’s regulation on the concentration of soil in soils used for cultivation of some plants. According to the joint regulation of WH and FAO, the maximum safe concentration of nickel in soil used for cultivation is 50 mg/kg and according to EU regulation, the level of nickel in normal (heath) soil is 75mg/kg. One can conclude that the soil sample was collected from Guagusa, Shikudad, and Banja agricultural areas were highly polluted by nickel accumulations, but that collected from Fagita Lecoma site had lower concentration relation with EU regulation and is not much exaggerated amount. The effect of Nickel on Fagita Lecoma soil is very limited. The major sources of nickel contamination in the soil are metal plating industries, combustion of fossil fuels for the preparation of charcoals.16 The accumulation may also be caused due to the application of manures and biosolids because the application of numerous biosolids (e.g., livestock manure, compost, and municipal sewage sludge) to the land inadvertently leads to the accumulation of heavy metals specially Nickel.17
As shown in the Figure 1 bar graph, the red bar means the soil sample collected from Guagusa Shikudad is highest for the regulated as well as the rest of soil samples. The soil samples collected from this district were less than that of the regulated amount by European Union (EU), but it is higher than that of the maximum safe limits regulated by the World Health Organization (WHO) and Food and Agricultural Organizations (FAO). Relatively, the agricultural sites of Guagusa Shikudad have high copper metal accumulation than other sites. In general, the concentrations of copper in the studied soil samples were high and it may affect the growth of potato and other plants. Wide application of various types of pesticides and fertilizers may be contributed to the increased availability of Cu in the soil.
From Figure 2, the red colored bar graph shows the highest concentration of zinc in the soil collected from Banja agricultural soil samples than the other ones. However, it is lower than the permissible level.18 The high level of Zn in these agricultural sites could probably be also due to the widely used vegetable farming activities in these areas and the high usage of various types of pesticides and fertilizers.
It can be seen from Figure 3 that in the red bar graph the concentrations of cadmium in soil samples collected from Guagusa Shikudad agricultural district have very high level. From the experimental data, the revealed data indicated that the concentrations of cadmium in all three agricultural sites have shown the high accumulation of cadmium and there is a direct impact on the cultivation of potato. This is because that the levels of cadmium in soil samples collected from the listed sites were higher than the normal regulated levels by WHO/FAO and EU.
In comparison with the safe limits of lead accumulation in soil, it is lower than that of the concentration of lead in soil regulated by WHO/FAO jointly and EU regulations. The content of lead metal does not affect the soil needed for potato cultivation because it is below the safe limit (Figure 4).
In general, the concentration of nickel in soil samples collected from three agricultural sites revealed high accumulation. As can be revealed in Figure 5, the accumulation and increased availability of Ni in the soil especially in Banja agricultural district may also be due to the wide application of various types of pesticides and fertilizers.
Figure 6 The graph of selected five heavy metals versus the concentration of Guagusa Shikudad (blue colour), Banja (red Colour) and Fagita lecoma (pale green).
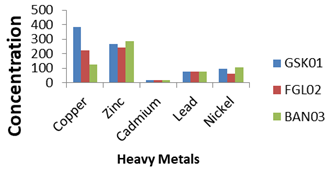
Figure 6 The graph of selected five heavy metals versus the concentration of Guagusa Shikudad (blue colour), Banja (red Colour) and Fagita lecoma (pale green).
In general, the results of the heavy metals analyzed in the study areas showed that their concentration level is below the standard guidelines for the maximum limit proposed for agricultural soils except in the case of copper and nickel as in Figure 6. Even although these heavy metal concentrations fell below the critical permissible concentration level of most heavy metals, it seems that their persistence in the soils of the study site may lead to increased uptake of these heavy metals by plants.
From the graph in Figure 6, the two metals mean copper and zinc have a high concentration in the soil, but cadmium, lead, and nickel have a high concentration in the soil. In comparing the selected metals in general, the highest content in the soil is copper and the lowest one of the metals is cadmium. As can be seen on Table 5, the orders of decreasing concentration of such metals are Cd<Pb<Ni<Zn<Cu. However, Copper, cadmium and nickel are above the safe level whereas, zinc and lead concentrations are safe for agricultural purposes.
|
Heavy metals |
Study area |
||
|
Fagita lecoma |
Banja |
Guagusa shikuda |
|
|
Cu |
Medium |
Least |
High |
|
Zn |
Least |
High |
Medium |
|
Cd |
Medium |
Least |
High |
|
Pb |
Least |
Medium |
High |
|
Ni |
Least |
High |
Medium |
Table 5 Relative comparison between three agricultural sites
It can be seen that from the three selected agricultural sites, Guagusa Shikudad agricultural soil contains the highest metal concentration of the rest. More specifically, Figure 3 shows that copper content is lower than the maximum limit level set by EU in Banja soil and higher than the limits in both Guagusa Shikudad and Fagita lecoma soil samples. And the contents of nickel and zinc were the lowest in Fagita lecoma soil samples.
The study indicates that soils serve as the potential source of heavy metals in the environment and the concentration of heavy metals: Pb, Cd, Ni, Cu, and Zn are some far below the maximum tolerable levels set by FAO/WHO, but the values of Cu, Cd, and Ni were higher than the maximum tolerable levels set by FAO/WHO. The level of contamination of the soil by heavy metals is high at present and the soil is polluted by toxic heavy metals (Cu, Cd, and Ni). The present study will give brief information about the heavy metal contents of the soil, and these results may serve as baseline data for the determination of mineral contents and physicochemical properties of the soil in the study area.
None.
None.
The authors declare that there is no conflict of interest.

©2021 Adamu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.