MOJ
eISSN: 2573-2919


Research Article Volume 3 Issue 3
1Department of Botany, Plant Physiology and Genetics, Republic of Tajikistan, Tajikistan
2Department of Botany, University of Peshawar, Pakistan Institute of Evolution, University of Haifa, Israel
Correspondence: Barinova SS, Institute of Evolution, University of Haifa, Mount Carmel, 199 Abba Khoushi Ave, Haifa, 3498838, Israel
Received: February 01, 2018 | Published: May 2, 2018
Citation: Niyatbekov T, Barinova SS. Bioindication of aquatic habitats with diatom algae in the Pamir Mountains, Tajikistan. MOJ Eco Environ Sci. 2018;3(3):117-120. DOI: 10.15406/mojes.2018.03.00075
Paper represents the first results of bioindication analysis of water quality in the Pamir aquatic habitats including rivers, lakes, mineral and hot springs with help of diatom algae. Altogether 438 bioindicators taxa were revealed from 558 known diatom algae in Pamir. Twelve environmental properties were assessed with help of species autecology. Bioindication analysis was doing for environmental parameters of water temperature, pH, salinity, and oxygen saturation as well as was assessed substrate preferences and nutrition type of diatoms, trophic state of water bodies and water quality on the base of organic pollution indications. We was find that diatoms in Pamir are strongly autotrophes, benthic or plankto–benthic inhabitants that preferred low–saline, low–alkaline, middle oxygenated clear fresh water with low organical pollution and oligo–to meso–eutrophic state. Compative floristics of Pamir and surrounding diatom floras let us to assume that studied algal flora can represent regional reference area for close related high mountains habitats.
Keywords: diatoms, algal flora, bioindication, pamir, tajikistan
The ecological assessments of environmental variables ranges that can characterize inhabited water body, river basin or even region are progressive and economic methods that implemented for many countries in monitoring, and one of them is algal bioindication.1 Diversity of algae in Tajikistan has been studied sporadically during the last century. The uppermost part of Tajikistan territory is Pamir where the large regional rivers Panj and Gunt are started. The rivers and streams in Pamir are placed in high mountains and started from glaciers. The altitude gradient of the Pamir aquatic habitats is represent of about two thousand meters and ranged between 2,000 and 4,000 m above the sea level (a.s.l.). This high mountain area is very rich in thermal and mineral waters, which in a way are unique habitats characterized by a constantly as well as high temperature from 10 ˚С to 86 ˚С, various chemical compositions and saturation by carbon dioxide, nitrogen gase and hydrogen sulphide. It classify as hydrogen sulfide–siliceous, hydrocarbonate–sulphate–calcium–magnesium, chloride–sulfate–calcium–sodium, hydrocarbonate–sulfate–sodium and weak radon–chloride–sulfate.2,3 In these waters, for many centuries, a special community of algae with a specific species composition and degree of species resistance to peculiarly extreme environmental conditions was formed and developed. Rivers and lakes are numerous in Pamir in altitude gradient. Therefore, Pamir is one of high altitude area in Eurasia with close relations to Hindu Kush, Altay, and Himalayas. Its territory have diverse aquatic habitats from clear freshwater large rivers, streams, lakes, to mineral and thermal springs which are occupied by diverse algal communities. Freshwater algae are widely used in ecological assessment of water quality.1 It is very important to know about algal diversity in inland waters because most of algal species can be used as environmental indicators. Usually, diatom algae represent about one–half of species richness in the well–studied middle–latitudinal regional algal floras.4 The references literature data on Pamir regional algal flora is known before our study from sporadically collected material in period 1930–1983.5–9 The regular work has been in 2000–2006 by G.R. Jumaeva.10 Our own study of diatoms in thermal and mineral springs is enriched the regional diversity by 134 diatom species (166 with infraspecific taxa).11 We assume that the diversity of this group of algae in Pamir is still far from complete. Altogether 558 taxa of diatom algae are known in Pamir aquatic habitats as a result of references collecting data and our own research.12 So, the list of species and studied waterbodies are representative for ecological characteristic of this highmountain territory. Thus, the aim of our work was to reveal bioindicator species in the full taxa list of diatom algae from different habitats of Pamir and to characterize of water quality on the base of species autecology by bioindication methods.
Sampling and laboratory studies
Our own material for this analysis is comes from 150 samples collected during few field trips in summer period of 2000–2015 from various thermal and mineral springs located at an altitude from 2,360 m to 3,800 m above the sea level.11 Algological samples were collected by scratching and scooping, placed in 15 ml plastic tubes, and partly fixed with 3% neutral formaldehyde solution, as well as partly not fixed and transported to the laboratory in the ice box. The diatoms shells were prepared by the peroxide technique9 modified for glass slides10 and were placed in the Naphrax® resin in two repetitions from each sample. The structure elements of the diatom shells were observed with Nikon stereomicroscope under magnifications 740x–1850x from two repetitions of each sample and were photographed with a DC in the Institute of Botany, Plant Physiology and Genetics, Dushanbe, Republic of Tajikistan, and the Institute of Evolution, University of Haifa. Diatom species were defined from permanent glass slides with help of international handbooks and its scientific names were validated with help of algaebase.org. Autecology of diatom taxa was taken from our database.13
Taxonomic data compilation
Total species list of the Pamir diatom flora was compiled in first time12 from our study of the mineral and thermal springs as well as from referenced lists in publications about diatom diversity in Pamir aquatic habitats.2,3,5–11 All collected taxonomic data was adapted to the modern taxonomic system with help of algaebase.org. Taxonomic list was analyzed in the Microsoft Access 2013 Program.
Diversity of diatom algae in pamir habitats
Altogether 558 taxa of species and subspecies levels has been revealed form the references data and our own study in Pamir.12 We studied earlier the influence of habitats altitude on species richness of freshwater algae and find that diversity is increased with increasing altitude14 up to two thousand meters a.s.l. Our list of diatom species in Pamir is comparable with richness of diatoms in close placed and well studied mountain regional floras such as South–Tajik Depression or Caucasus,15–17 but in the regions where algal diversity study are in initial stage like Kabul River basin in Pakistan18,19 the Pamir diversity of diatoms looks like much richer. Bioindication properties were found for 438 taxa of diatoms from Pamir aquatic habitats. The list of bioindicators contains about 78.4% of total species list of Pamir diatoms (Table 1). Therefore, the bioindicational analysis can be satisfy representative.
Bioindicational analysis of the Pamir environment based on diatom algae autecology
Bioindicators of Pamir environment were revealed for ten ecological variables: substrate preferences, water oxygen saturation, water pH, salinity, organic pollution related to self–purification zones and Indices of saprobity, trophic level of ecosystem habitat, and type of nutrition of diatom cells (Table 1). Can be seen, that substrate preferences are the property of species, which mostly represented in the Pamir diatom flora (428). Salinity preferences are known for 411 taxa, and water pH for 390. Organic pollution indicators are represented by more than 300 taxa. Important for aquatic inhabitants’ property, the water oxygen saturation can be accessed on the base of 261 indicator taxa. Less known property of diatoms is the water temperature preferences. Nevertheless, the temperature preference is known for 89 diatoms species in Pamir. We were constructed the distribution of indicator taxons of each of the environmental indicators over trend of amplifying the value of the indicator group for the revealing prevailed groups of indicators. Standard deviation line was added to each distribution for cut off the dominant groups. The trend line also was constructed to show each variables tendency in Pamir. The distributions were constructed for indicator groups as the environmental property increases. So, three ecological groups were found in 428 substrate preferences indicators in the Pamir diatom flora (Figure 1). Trend line of distribution show sharp increasing of benthic group in species number. The STDEV line, also added to most ecologically important groups the planktonic–benthic group of species that preferred slowly streaming waters. Temperature preferences indicators are divided into four ecological groups for Pamir. Can be seen (Figure 1) that temperate water temperature species are prevail, but important also that cool–water species and warm–water indicators are visible in distribution but cannot be included in the dominant groups as shows the STDEV line.
Indicators of water oxygen saturation reflect the predominance of slowly streaming waters group (Figure 1), but species preferring streaming waters also were numerous and over the STDEV line. Water pH indicators were represented by six ecological groups with prevailing of indifferent and alkaliphilic species as can be seen with trend and STDEV lines (Figure 1). Water salinity indication reflect fresh and low–saline waters in Pamir (Figure 2) as is shown by trend and STDEV lines, although the wide amplitude of indicators is presented from halophobes to mesohalobes. Organic pollution indication by diatom species according T. Watanabe (Figure 2) show prevailence of middle–polluted waters group with the trend line, but with STDEV line can be seen in dominant groups the saproxenes also, that are indicators of fresh clear natural waters. Seven groups of trophic state indicators were revealed in Pamir diatom flora (Figure 2) in which tendency to oligotrophic groups can be seen with trend line. Nevertheless, STDEV line shows two different groups of trophic indicators related to not only oligotrophic waters (on left in Figure 2) but also to mesotrophic and eutrophic waters (on right in Figure 2). Therefore, the indicators distribution reflects the different trophic state of the Pamir water bodies from oligotrophic to eutrophic. The distribution of Pamir diatom indicators over the nutrition types properties of organisms show strongly prevailing of autotrophs that preferred photosynthetic way of nutrition in the ecosystems (Figure 2). Assessment of organic pollution was doing with help of grouping of saprobity indicators to major Water Quality Classes based on the species–specific saprobity according Sládeček. Figure 2 show that diatoms in Pamir are divided into four water quality Classes that reflects the waters from natural clear to significantly polluted. The colors in the Class of Water Quality histogram are as in EU color code. This distribution shows Classes 2 and 3 prevailing with both, trend and STDEV lines. Pamir environment bioindicational analysis show similar results with other high mountain regions such as Caucasus16 where mountain part of territory has minimal anthropogenic influence. In contrary, even in close related South–Tadjik Depression algal flora15 we can see of anthropogenic influence doing mostly from agriculture. In such low studied regions with high agricultural and other human activity, as the Kabul River valley with tributaries from Hindu Kush to Peshawar,18,19 can be seen decreasing of algal species richness with increasing of water pollution by salinity and organic matters. Therefore, the Pamir diatom algal flora can represent the regional background diversity for comparing of closely spaced high mountain habitats.
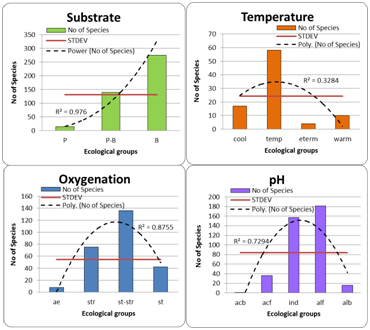
Figure 1 Distribution of species indicators of the diatom Pamir algal flora over the ecological groups of substrate preferences, temperature, oxygen saturation, and water pH. The ecological categories abbreviations as in Table 1.
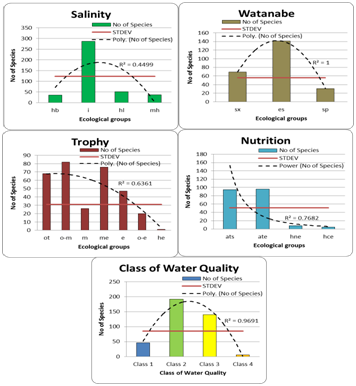
Figure 2 Distribution of species indicators of the diatom Pamir flora over the ecological groups of salinity, organic pollution according Watanabe, trophic state, nutrition type, and water quality Class based on the species–specific saprobity according Sládeček. The colors in the Class of Water Quality histogram are in EU color code. The ecological categories abbreviations as in Table 1.
No. |
Taxa |
Hab |
T |
Oxy |
pH |
Sal |
D |
Sap |
S |
Tro |
Aut–Het |
1 |
Achnanthes brevipes C Agardh var. brevipes |
B |
– |
– |
alf |
hl |
– |
b |
2 |
me |
– |
2 |
Achnanthes brevipes var. intermedia (Kützing) Cleve |
B |
– |
st |
– |
mh |
– |
– |
– |
– |
– |
3 |
Achnanthes coarctata (Brébisson ex W. Smith) Grunow in Cleve and Grunow |
B |
– |
ae |
ind |
hl |
– |
x |
0.2 |
ot |
– |
4 |
Achnanthes conspicua var. brevistriata Hustedt |
B |
– |
– |
ind |
i |
sx |
o |
1 |
– |
– |
5 |
Achnanthes dispar var. angustissima (Jasnitsky) Sheshukova in Proschkina–Lavrenko |
B |
– |
– |
– |
hb |
– |
– |
– |
– |
– |
6 |
Achnanthes exigua Grunow in Cleve & Grunow |
B |
eterm |
st–str |
alf |
i |
sp |
b |
2 |
o–e |
ate |
7 |
Achnanthidium exile (Kützing) Heiberg |
B |
– |
str |
alb |
i |
sx |
o–a |
1.8 |
o–m |
ats |
8 |
Achnanthidium gracillimum (Meister) Lange–Bertalot |
B |
– |
– |
alf |
– |
– |
o–x |
0.7 |
– |
– |
9 |
Achnanthidium lanceolatum var. ventricosum (Hustedt) Poretsky |
B |
warm |
– |
alf |
i |
sx |
x–b |
0.8 |
– |
– |
10 |
Achnanthidium lineare W Smith |
P–B |
– |
– |
ind |
i |
es |
o–b |
1.5 |
me |
– |
11 |
Achnanthidium minutissimum (Kützing) Czarnecki |
P–B |
eterm |
st–str |
ind |
i |
es |
x–b |
0.95 |
o–e |
ate |
12 |
Achnanthidium minutum Cleve |
B |
– |
– |
acf |
hb |
– |
– |
– |
– |
– |
13 |
Actinella punctata FW Lewis |
B |
– |
– |
acf |
hb |
– |
o |
1 |
ot |
– |
14 |
Adlafia bryophila (Petersen JB) Lange–Bertalot in Gerd Moser, Lange–Bertalot & Metzeltin |
P–B, aer |
– |
st–str |
acf |
– |
– |
o–x |
0.7 |
ot |
– |
15 |
Adlafia minuscula (Grun.) Lange–Bertalot var. minuscula |
P–B |
– |
st–str |
ind |
– |
es |
a–o |
2.8 |
ot |
– |
16 |
Adlafia minuscula var. muralis (Grunow) Lange–Bertalot in Lange–Bertalot & Genkal |
B |
– |
– |
– |
– |
sp |
a–o |
2.8 |
– |
– |
17 |
Amphora commutata Grunow in Van Heurck |
B |
– |
– |
– |
hl,mh |
– |
– |
– |
e |
– |
18 |
Amphora libyca Ehrenberg |
B |
temp |
st |
alf |
i |
es |
o–b |
1.5 |
o–m |
– |
19 |
Amphora mongolica Østrup var. mongolica |
B |
– |
– |
ind |
i |
– |
– |
– |
– |
– |
20 |
Amphora ovalis (Kützing) Kützing var. ovalis |
B |
temp |
st–str |
alf |
i |
sx |
o–b |
1.5 |
me |
ate |
21 |
Amphora ovalis var. gracilis (Ehrenberg) Van Heurck |
B |
– |
– |
alf |
i |
sx |
o–b |
1.5 |
– |
– |
22 |
Amphora pediculus (Kütz.) Grun. ex A. Schmidt |
B |
temp |
st |
alf |
i |
es |
b–o |
1.7 |
o–m |
ate |
23 |
Aneumastus apiculatus (Østrup) Lange–Bertalot in Lange–Bertalot & Genkal |
P–B |
– |
– |
acf |
– |
– |
b |
2 |
ot |
– |
24 |
Aneumastus minor Lange–Bertalot |
P–B |
– |
– |
alf |
i |
– |
o |
1.2 |
o–m |
– |
25 |
Aneumastus rostratus (Hustedt) Lange–Bertalot |
P–B |
– |
– |
alf |
i |
– |
b |
2 |
– |
– |
26 |
Aneumastus tuscula (Ehrenberg) Mann DG & Stickle in Round AJ, Crawford RM & Mann DG |
P–B |
– |
– |
alf |
i |
– |
x–b |
0.9 |
o–e |
– |
27 |
Anomoeoneis costata (Kützing) Hustedt |
B |
– |
– |
– |
mh |
– |
a–o |
2.7 |
– |
– |
28 |
Anomoeoneis sphaerophora Pfitzer var. sphaerophora |
B |
warm |
st–str |
alf |
hl |
– |
a–o |
2.7 |
me |
ate |
29 |
Anomoeoneis sphaerophora var. guentheri Otto Müller |
B |
– |
– |
acf |
hl |
– |
– |
– |
– |
– |
30 |
Anomoeoneis sphaerophora var. sculpta (Ehrenberg) Otto Müller |
B |
– |
– |
– |
mh |
– |
a–o |
2.7 |
– |
– |
31 |
Aulacoseira alpigena (Grunow) Krammer |
P–B |
cool |
st–str |
alf |
i |
sp |
x–b |
0.8 |
ot |
ats |
32 |
Aulacoseira distans (Ehrenberg) Simonsen |
P–B |
cool |
str |
acf |
i |
sp |
x–o |
0.4 |
ot |
ats |
33 |
Aulacoseira granulata (Ehrenberg) Simonsen var. granulata |
P–B |
temp |
st–str |
ind |
i |
es |
b |
2 |
me |
ate |
34 |
Aulacoseira granulata var. angustissima (Müll O) Simons. |
P |
temp |
st–str |
ind |
i |
es |
b |
2.1 |
o–m |
ate |
35 |
Aulacoseira italica (Ehrenberg) Simonsen |
P–B |
cool |
st–str |
ind |
i |
es |
o–b |
1.45 |
me |
– |
36 |
Aulacoseira valida (Grunow) Krammer |
P–B |
– |
– |
ind |
i |
es |
o |
1.3 |
o–m |
– |
37 |
Bacillaria paxillifera (Müller OF) Marsson T |
P–B |
– |
– |
ind |
hl |
es |
b |
2.3 |
me |
ate |
38 |
Brachysira exilis (Kützing) Round & Mann DG |
B |
– |
– |
alb |
hl |
es |
x–b |
0.8 |
ot |
– |
39 |
Brachysira microcephala (Grunow) Compère |
B |
– |
– |
– |
i |
sx |
o |
1 |
– |
– |
40 |
Brachysira serians (Brébisson) Round & Mann DG |
B |
– |
st–str |
acf |
hb |
– |
x |
0.2 |
ot |
ats |
41 |
Brebissonia lanceolata (Agardh) Mahoney and Reimer |
P–B |
temp |
– |
alf |
i |
– |
x–o |
0.4 |
– |
– |
42 |
Caloneis amphisbaena (Bory) Cleve |
B |
– |
st–str |
alf |
i |
– |
b |
2.3 |
me |
ate |
43 |
Caloneis bacillum (Grunow) Cleve |
B |
temp |
st–str |
ind |
i |
es |
o |
1.3 |
me |
ats |
44 |
Caloneis limosa (Kützing) R.M.Patrick in Patrick & Reimer |
P–B |
– |
st–str |
alf |
i |
es |
o |
1.1 |
m |
ats |
45 |
Caloneis molaris (Grunow) Krammer in Krammer & Lange–Bertalot |
B |
– |
str |
ind |
i |
es |
o |
1 |
ot |
– |
46 |
Caloneis schumaniana (Grunow) Cleve var. schumaniana |
P–B |
– |
st–str |
ind |
i |
es |
x–b |
0.9 |
o–m |
ats |
47 |
Caloneis silicula (Ehrenberg) Cleve var. silicula |
B |
– |
st |
ind |
i |
sp |
o |
1.3 |
o–m |
ats |
48 |
Caloneis silicula var. jenissejensis Grunow |
B |
– |
st |
acf |
hl |
– |
– |
– |
– |
– |
49 |
Caloneis silicula var. kjellmaniana Cleve |
B |
– |
– |
alb |
i |
– |
– |
– |
– |
– |
50 |
Caloneis tenuis (Gregory) Krammer |
B |
– |
str |
neu |
i |
– |
x |
0.2 |
ot |
ats |
51 |
Caloneis undulata (Gregory) Krammer |
B |
– |
st |
acf |
i |
– |
o |
1 |
ot |
ats |
52 |
Caloneis ventricosa (Ehrenberg) F. Meister var. ventricosa |
B |
– |
st |
alf |
i |
sp |
o |
1.3 |
– |
– |
53 |
Caloneis ventricosa var. truncatula (Grunow) Meister |
B |
– |
– |
– |
– |
– |
o |
1.3 |
– |
– |
54 |
Campylodiscus clypeus (Ehrenberg) Ehrenberg ex Kützing |
B |
temp |
– |
alb |
mh |
– |
b |
2 |
e |
– |
55 |
Cavinula cocconeiformis (Gregory ex Greville) Crawford & Mann |
P–B |
– |
str |
ind |
i |
es |
x–o |
0.4 |
o–m |
ats |
56 |
Cavinula scutelloides (Smith W) Lange–Bertalot in Lange–Bertalot & Metzeltin |
B |
– |
– |
– |
I |
– |
o–b |
1.5 |
me |
– |
57 |
Cocconeis disculus (Schumann) Cleve in Cleve & Jentzsch |
B |
– |
st |
alf |
i |
es |
o–x |
0.7 |
me |
– |
58 |
Cocconeis neodiminuta Krammer |
P–B |
temp |
st–str |
alf |
i |
sx |
x–b |
0.9 |
me |
– |
59 |
Cocconeis pediculus Ehrenberg |
B |
– |
st–str |
alf |
i |
sx |
o–a |
1.8 |
me |
ate |
60 |
Cocconeis placentula Ehrenberg var. placentula |
P–B |
temp |
st–str |
alf |
i |
es |
o |
1.35 |
me |
ate |
61 |
Cocconeis placentula var. euglypta (Ehrenberg) Grunow |
P–B |
temp |
st–str |
alf |
i |
sx |
o |
1.3 |
o–m |
ate |
62 |
Cocconeis placentula var. intermedia (Héribaud–Joseph & Peragallo M) Cleve |
B |
– |
st–str |
alf |
i |
– |
o–b |
1.4 |
ot |
ate |
63 |
Cocconeis placentula var. lineata (Ehrenberg) Van Heurck |
P–B |
– |
st–str |
alf |
i |
sx |
o |
1.2 |
o–m |
ate |
64 |
Cocconeis placentula var. rouxii (Héribaud–Joseph & Brun) Cleve |
B |
– |
– |
alf |
i |
– |
o–b |
1.4 |
– |
– |
65 |
Cocconeis scutellum Ehrenberg |
B |
– |
– |
alf |
hl |
– |
b |
2 |
me |
– |
66 |
Cosmioneis pusilla (Smith W) Mann DG & Stickle in Round AJ, Crawford & Mann |
P–B, aer |
– |
str |
ind |
hl |
sp |
o–a |
1.8 |
o–m |
ats |
67 |
Craticula ambigua (Ehrenberg) Mann in DG Round, Crawford & Mann DG |
B |
warm |
st |
alf |
i |
es |
b |
2.3 |
me |
– |
68 |
Craticula cuspidata (Kützing) Mann |
B |
temp |
st–str |
alf |
i |
es |
b–a |
2.45 |
me |
– |
69 |
Craticula halophila (Grunow) Mann DG in Round, Crawford RM & Mann DG |
B |
– |
st–str |
alf |
mh |
es |
a |
3 |
e |
ate |
70 |
Ctenophora pulchella (Ralfs ex Kützing) DM Williams et Round var. pulchella |
P–B |
– |
st–str |
alf |
i |
– |
b |
2.3 |
o–m |
ate |
71 |
Ctenophora pulchella var. lacerata (Hustedt) Bukhtiyarova |
B |
– |
– |
– |
mh |
– |
– |
– |
– |
– |
72 |
Cyclotella choctawhatcheeana Prasad in Prasad, Neinow & Livingston |
P |
– |
– |
– |
hl |
– |
– |
– |
– |
– |
73 |
Cyclotella meneghiniana Kützing |
P–B |
temp |
st |
alf |
hl |
sp |
a–o |
2.8 |
e |
hne |
74 |
Cyclotella radiosa (Grunow) Lemmermann |
P |
– |
st–str |
alb |
i |
sx |
o |
1.2 |
o–m |
ats |
75 |
Cymatopleura elliptica (Brébisson) Smith W |
P–B |
– |
st–str |
alf |
i |
– |
o–b |
1.4 |
me |
ate |
76 |
Cymbella affinis Kützing |
B |
temp |
st–str |
alf |
i |
sx |
o |
1.1 |
ot |
ats |
77 |
Cymbella amplificata Krammer |
P–B |
– |
– |
neu |
hb |
– |
o |
1 |
ot |
– |
78 |
Cymbella aspera (Ehrenberg) Cleve var. aspera |
B |
– |
st–str |
neu |
i |
es |
x |
0.3 |
o–e |
ats |
79 |
Cymbella cistula (Ehrenberg) Kirchner O var. cistula |
B |
– |
st–str |
alf |
i |
sx |
o |
1.2 |
e |
ats |
80 |
Cymbella compacta Østrup |
B |
– |
– |
– |
– |
– |
o–x |
0.7 |
– |
– |
81 |
Cymbella cymbiformis Agardh C |
B |
temp |
str |
ind |
i |
sx |
b |
2 |
o–m |
ats |
82 |
Cymbella falaisensis (Grunow) Krammer & Lange–Bertalot |
B |
– |
str |
– |
hb |
es |
o |
1 |
o–m |
ats |
83 |
Cymbella helvetica Kützing var. helvetica |
B |
– |
str |
ind |
i |
– |
o–x |
0.6 |
o–m |
– |
84 |
Cymbella helvetica var. curta Cleve |
B |
– |
– |
alf |
i |
– |
– |
– |
– |
– |
85 |
Cymbella hustedtii Krasske |
B |
– |
str |
neu |
i |
– |
o |
1 |
o–m |
ats |
86 |
Cymbella laevis Nägeli in Rabenhorst |
B |
cool |
– |
ind |
i |
sx |
– |
– |
– |
– |
87 |
Cymbella lanceolata (Agardh C) C Agardh var. lanceolata |
B |
– |
st–str |
alf |
i |
es |
b–a |
2.5 |
e |
ate |
88 |
Cymbella lanceolata var. notata Wisloukh & Poretzky |
B |
– |
– |
– |
hl |
– |
– |
– |
– |
– |
89 |
Cymbella neocistula Krammer |
B |
– |
– |
ind |
I |
– |
o |
1.2 |
o–m |
– |
90 |
Cymbella obtusiuscula Kützing |
B |
– |
– |
ind |
i |
– |
o |
1 |
– |
– |
91 |
Cymbella parva (Smith W) Kirchner |
B |
– |
– |
ind |
I |
– |
b |
2 |
o–m |
– |
92 |
Cymbella stuxbergii (Cleve) Cleve |
B |
– |
– |
neu |
i |
– |
o |
1 |
ot |
– |
93 |
Cymbella tumida (Brébisson) Van Heurck |
B |
temp |
str |
alf |
i |
sx |
b |
2.2 |
me |
ats |
94 |
Cymbella turgidula Grunow in Schmidt A et al. |
B |
– |
st–str |
ind |
– |
es |
– |
– |
– |
– |
95 |
Cymbellа tartuensis Molder |
B |
– |
– |
ind |
i |
– |
– |
– |
– |
– |
96 |
Cymbopleura amphicephala (Naegeli) Krammer |
B |
– |
str |
ind |
i |
sx |
o |
1.2 |
o–m |
ats |
97 |
Cymbopleura anglica (Lagerstedt) Krammer |
B |
– |
– |
ind |
hb |
– |
o |
1 |
ot |
– |
98 |
Cymbopleura angustata (Smith W) Krammer |
B |
– |
str |
ind |
i |
es |
o |
1 |
ot |
ats |
99 |
Cymbopleura hybrida (Grunow ex Cleve) Krammer |
P–B |
– |
– |
ind |
hl |
– |
o |
1 |
o–m |
– |
100 |
Cymbopleura inaequalis (Ehrenberg) Krammer |
B |
– |
st–str |
ind |
i |
– |
o |
1.1 |
m |
ats |
101 |
Cymbopleura incerta (Grunow) Krammer |
P–B |
– |
str |
ind |
hb |
– |
o |
1 |
ot |
ats |
102 |
Cymbopleura naviculiformis (Auerswald ex Heiberg) Krammer |
B |
– |
st–str |
ind |
i |
es |
o |
1.2 |
o–m |
ate |
103 |
Cymbopleura reinhardtii (Grunow) Krammer K |
B |
– |
str |
ind |
i |
sx |
o |
1 |
m |
ats |
104 |
Cymbopleura subaequalis (Grunov) Krammer |
B |
– |
str |
neu |
hb |
sx |
o |
1 |
o–m |
ats |
105 |
Cymbopleura subcuspidata (Krammer) Krammer |
P–B |
– |
str |
acf |
i |
– |
o |
1 |
ot |
ats |
106 |
Delicata delicatula (Kützing) Krammer |
B, aer |
– |
str |
alf |
i |
sx |
o |
1 |
ot |
ats |
107 |
Denticula kuetzingii Grunow |
P–B |
– |
str |
ind |
i |
es |
x–b |
0.9 |
m |
– |
108 |
Denticula tenuis Kützing |
B |
– |
str |
alf |
i |
sx |
x |
0.3 |
m |
ats |
109 |
Denticula thermalis Kützing |
B |
warm |
– |
alf |
– |
– |
b |
2 |
me |
– |
110 |
Diadesmis contenta var. biceps (Grunow) P.B.Hamilton in Hamilton et al. |
B |
– |
– |
– |
– |
sx |
o–x |
0.7 |
– |
– |
111 |
Diatoma elongata (Lyngbye) Agardh C |
P–B |
– |
st–str |
ind |
hl |
sx |
o |
1.3 |
e |
ate |
112 |
Diatoma moniliformis (Kützing) Williams DM |
P–B |
– |
st–str |
alf |
i |
– |
o |
1.3 |
o–m |
– |
113 |
Diatoma vulgaris Bory var. vulgaris |
P–B |
– |
st–str |
ind |
i |
sx |
b |
2.2 |
me |
ate |
114 |
Diatoma vulgaris var. brevis Grunow |
P–B |
– |
st–str |
alb |
i |
sx |
b |
2.2 |
me |
ate |
115 |
Diatoma vulgaris var. linearis Grunow in Van Heurck |
B |
– |
str |
alf |
i |
es |
b |
2.2 |
me |
ate |
116 |
Diatoma vulgaris var. producta Grunow |
B |
– |
st–str |
alf |
i |
– |
b |
2.2 |
me |
– |
117 |
Didymosphenia geminata (Lyngbye) Mart.Schmidt in A.Schmidt |
B |
– |
st–str |
ind |
i |
sx |
o–x |
0.7 |
ot |
– |
118 |
Diploneis elliptica (Kützing) Cleve |
B |
temp |
str |
alf |
i |
sx |
o–x |
0.6 |
m |
ats |
119 |
Diploneis oblongella (Nägeli ex Kützing) Cleve–Euler |
B |
– |
str |
ind |
i |
sx |
x–b |
0.9 |
ot |
ats |
120 |
Diploneis oculata (Brébisson) Clve |
B |
– |
st |
ind |
i |
– |
x–b |
0.9 |
– |
– |
121 |
Diploneis ovalis (Hilse) Cleve |
B |
– |
str |
alf |
i |
sp |
x–b |
0.9 |
o–m |
ats |
122 |
Diploneis parma Cleve |
B |
cool |
– |
alf |
i |
– |
o–b |
1.4 |
ot |
– |
123 |
Diploneis subovalis Cleve |
B |
temp |
st–str |
ind |
i |
– |
x–o |
0.5 |
ot |
– |
124 |
Discostella stelligera (Cleve and Grunow) Houk and Klee |
P |
– |
– |
ind |
I |
– |
o–b |
1.4 |
o–m |
– |
125 |
Ellerbeckia arenaria (Moore ex Ralfs) Crawford |
P–B |
cool |
st–str |
alf |
i |
– |
o–x |
0.6 |
ot |
ats |
126 |
Encyonema alpinum (Grunow) Mann in Round DG, Crawford RM & Mann DG |
B |
– |
str |
ind |
hb |
es |
x–o |
0.5 |
ot |
ats |
127 |
Encyonema caespitosum Kützing |
B |
– |
– |
– |
i |
sx |
o |
1.3 |
o–e |
– |
128 |
Encyonema elginense (Krammer) Mann in Round DG, Crawford & Mann |
B |
temp |
st |
acf |
hb |
sx |
o–b |
1.5 |
– |
– |
129 |
Encyonema gracile Rabenhorst |
B |
– |
– |
ind |
hb |
sx |
x |
0.3 |
– |
– |
130 |
Encyonema lacustre (Agardh C) Pantocsek |
B |
– |
– |
ind |
hl |
sx |
b–a |
2.4 |
me |
– |
131 |
Encyonema minutum (Hilse) Mann DG |
B |
– |
st–str |
ind |
i |
sx |
o |
1.2 |
o–e |
ate |
132 |
Encyonema prostratum (Berkeley) Kützing |
P–B |
– |
str |
alb |
i |
es |
o |
1.3 |
e |
ats |
133 |
Encyonema silesiacum (Bleisch in Rabenhorst) Mann DG |
B |
– |
st–str |
ind |
i |
sx |
o |
1.2 |
o–e |
ate |
134 |
Encyonopsis microcephala (Grunow) Krammer |
B |
– |
str |
alf |
i |
es |
o |
1.3 |
me |
ats |
135 |
Entomoneis alata (Ehrenberg) Ehrenberg |
P–B |
– |
st |
alf |
mh |
– |
b |
2 |
– |
– |
136 |
Entomoneis ornata (Bailey) Reimer |
B |
– |
st–str |
alf |
i |
– |
o–b |
1.5 |
o–m |
ats |
137 |
Entomoneis paludosa (Smith W) Reimer var. paludosa |
P–B |
– |
– |
alf |
hl |
– |
b–a |
2.5 |
m |
– |
138 |
Epithemia adnata (Kützing) Brébisson var. adnata |
B |
temp |
st |
alb |
i |
sx |
o |
1.2 |
me |
ats |
139 |
Epithemia adnata var. porcellus (Kützing) Ross R |
B |
– |
– |
alf |
i |
– |
b |
2 |
me |
– |
140 |
Epithemia adnata var. saxonica (Kützing) Patrick RM in Patrick & Reimer |
B |
temp |
– |
alf |
i |
– |
o |
1.2 |
me |
– |
141 |
Epithemia argus (Ehrenberg) Kützing var. argus |
P–B |
– |
st–str |
ind |
i |
es |
o–x |
0.7 |
m |
– |
142 |
Epithemia argus var. alpestris (Smith W) Grunow |
B |
– |
– |
ind |
i |
– |
o–x |
0.7 |
m |
– |
143 |
Epithemia argus var. angusta Tarnavschi |
B |
– |
– |
ind |
i |
– |
– |
– |
– |
– |
144 |
Epithemia argus var. longicornis (Ehrenberg) Grunow |
P–B |
– |
– |
ind |
i |
– |
– |
– |
– |
– |
145 |
Epithemia sorex Kützing |
B |
temp |
st–str |
alf |
i |
sx |
o |
1.1 |
me |
ats |
146 |
Epithemia turgida (Ehrenberg) Kützing var. turgida |
B |
temp |
st |
alf |
i |
sx |
x–b |
0.9 |
me |
ats |
147 |
Epithemia turgida var. capitata Fricke in Schmidt et al. |
B |
– |
– |
alf |
i |
– |
– |
– |
– |
– |
148 |
Epithemia turgida var. granulata (Ehrenberg) Brun |
B |
– |
st |
alf |
I |
– |
x–b |
0.9 |
o–m |
ats |
149 |
Eucocconeis flexella (Kützing) Meister |
B |
– |
str |
ind |
mh |
sx |
x |
0.2 |
ot |
ats |
150 |
Eucocconeis quadratarea (Østrup) Lange–Bertalot & Genkal |
B |
– |
– |
acf |
i |
sx |
x |
0.3 |
– |
– |
151 |
Eunotia arcus Ehrenberg |
B |
– |
st–str |
acf |
i |
– |
x–o |
0.5 |
ot |
ats |
152 |
Eunotia bidens Ehrenberg |
P–B |
cool |
str |
acf |
hb |
– |
x–o |
0.4 |
o–m |
ats |
153 |
Eunotia bilunaris (Ehrenberg) Scharschmidt |
B |
temp |
st–str |
acf |
i |
es |
o |
1 |
o–e |
ate |
154 |
Eunotia diodon Ehrenberg |
B |
cool |
st |
acf |
i |
– |
x |
0.2 |
ot |
ats |
155 |
Eunotia exigua (Brébisson in Kützing) Rabenhorst |
P–B |
– |
st–str |
acf |
hb |
es |
x–o |
0.45 |
o–e |
ate |
156 |
Eunotia faba Ehrenberg |
B |
temp |
st–str |
acf |
i |
sx |
o |
1.1 |
o–m |
ats |
157 |
Eunotia pectinalis (Kützing) Rabenhorst |
B |
– |
str |
ind |
I |
sx |
x |
0.3 |
m |
ate |
158 |
Eunotia polydentula (Brun) Hustedt |
B |
– |
– |
acf |
hb |
– |
x–o |
0.5 |
– |
– |
159 |
Eunotia praerupta Ehrenberg |
P–B |
cool |
st–str |
acf |
I |
sx |
x–o |
0.4 |
o–m |
ats |
160 |
Eunotia valida Hustedt |
P–B |
– |
– |
acf |
hb |
– |
x–o |
0.5 |
ot |
– |
161 |
Fallacia insociabilis (Krasske) D.G.Mann in F.E.Round, R.M.Crawford & D.G.Mann |
B |
– |
ae |
neu |
hb |
es |
o |
1 |
m |
ats |
162 |
Fallacia pygmaea (Kützing) De Mann |
P–B |
– |
st–str |
alf |
mh |
es |
a–o |
2.7 |
e |
hne |
163 |
Fragilaria acus (Kützing) Lange–Bertalot in Krammer & Lange–Bertalot |
P |
– |
st–str |
alb |
i |
es |
o–a |
1.8 |
– |
– |
164 |
Fragilaria alpestris Krasske |
P–B, aer |
– |
– |
ind |
i |
– |
o |
1 |
ot |
– |
165 |
Fragilaria amphicephaloides Lange–Bertalot |
B |
– |
– |
alf |
i |
sp |
x–a |
1.55 |
o–m |
– |
166 |
Fragilaria bidens Heiberg |
P–B |
– |
str |
alf |
i |
– |
b |
2 |
e |
ats |
167 |
Fragilaria brevistriata Grunow in Van Heurck |
P–B |
– |
st–str |
alf |
i |
– |
o |
1.2 |
o–e |
ats |
168 |
Fragilaria capucina Desmazières var. capucina |
P–B |
– |
– |
ind |
i |
es |
b–o |
1.6 |
m |
– |
169 |
Fragilaria crotonensis Kitton |
P |
– |
st–str |
alf |
I |
es |
o–b |
1.5 |
m |
ate |
170 |
Fragilaria famelica (Kützing) Lange–Bertalot |
P–B |
– |
str |
alf |
i |
es |
o |
1.3 |
m |
ats |
171 |
Fragilaria gracilis Østrup |
P–B |
– |
– |
neu |
– |
– |
x–b |
0.8 |
o–m |
– |
172 |
Fragilaria inflata var. istvanffyi (Pantoscek) Hustedt |
B |
– |
str |
alf |
I |
– |
o |
1 |
me |
ats |
173 |
Fragilaria mesolepta Rabenhorst |
P–B |
– |
st–str |
alf |
i |
sx |
o–a |
1.9 |
– |
– |
174 |
Fragilaria radians (Kützing) Lange–Bertalot |
P–B |
– |
st–str |
alf |
I |
sx |
b–o |
1.7 |
o–m |
– |
175 |
Fragilaria recapitellata Lange H–Bertalot &Nergui D |
B |
– |
– |
– |
– |
sx |
– |
– |
– |
– |
176 |
Fragilaria rumpens GWF (Kützing) Carlson |
B |
– |
– |
alf |
i |
es |
x–a |
1.55 |
– |
– |
177 |
Fragilaria tenera (Smith W) Lange–Bertalot |
P–B |
– |
str |
acf |
hb |
sx |
b |
2.3 |
o–m |
ats |
178 |
Fragilaria vaucheriae (Kützing) J.B.Petersen |
P–B, Ep |
– |
st–str |
alf |
i |
sx |
o–a |
1.95 |
e |
ate |
179 |
Fragilariforma bicapitata (Mayer A) Williams DM & Round |
P–B |
– |
str |
ind |
hb |
– |
o |
1.3 |
o–e |
ats |
180 |
Fragilariforma virescens (Ralfs) Williams DM & Round |
P–B |
– |
st |
ind |
i |
es |
x–o |
0.4 |
o–m |
ats |
181 |
Fragilariopsis cylindrus (Grunow) Helmcke & Krieger |
P–B |
– |
– |
alf |
mh |
– |
o |
1 |
me |
– |
182 |
Frustulia rhomboides (Ehrenberg) De Toni |
B |
– |
st |
acf |
hb |
es |
x |
0.3 |
ot |
ats |
183 |
Gliwiczia calcar (Cleve) M.Kulikovskiy, Lange–Bertalot & Witkowski A |
B |
– |
– |
ind |
hb |
– |
o |
1 |
ot |
– |
184 |
Gomphoneis herculeana (Ehrenberg) Cleve |
B |
– |
– |
– |
hb |
sx |
o |
1 |
ot |
– |
185 |
Gomphonema acuminatum Ehrenberg var. acuminatum |
B |
– |
st |
ind |
i |
es |
o–b |
1.4 |
o–m |
ats |
186 |
Gomphonema acuminatum var. brebissonii (Kützing) Grunow in Van Heurck |
B |
– |
st |
ind |
i |
– |
b |
2 |
– |
– |
187 |
Gomphonema angustatum (Kützing) Rabenhorst |
B |
– |
st–str |
ind |
i |
es |
o |
1.3 |
o–m |
– |
188 |
Gomphonema calcareum Cleve |
B |
– |
st–str |
alf |
i |
– |
b |
2.3 |
o–m |
ate |
189 |
Gomphonema constrictum var. capitatum (Ehrenberg) Grunow |
B |
– |
– |
– |
i |
– |
– |
– |
– |
– |
190 |
Gomphonema coronatum Ehrenberg |
B |
– |
st |
ind |
i |
– |
o–b |
1.4 |
o–m |
– |
191 |
Gomphonema exilissimum (Grunow) Lange–Bertalot & Reichardt in Lange E –Bertalot & Metzeltin |
B |
– |
str |
ind |
i |
es |
o–x |
0.7 |
ot |
ats |
192 |
Gomphonema gracile Ehrenberg |
B |
temp |
st |
alf |
i |
es |
x–b |
0.8 |
m |
ats |
193 |
Gomphonema grunowii Patrick RM & Reimer |
B |
temp |
– |
alf |
i |
– |
b |
2 |
– |
– |
194 |
Gomphonema hedinii Hustedt |
B |
– |
– |
– |
– |
sx |
– |
– |
– |
– |
195 |
Gomphonema intricatum Kützing |
B |
– |
st–str |
ind |
i |
es |
o |
1.1 |
– |
– |
196 |
Gomphonema longiceps Ehrenberg |
B |
– |
str |
ind |
i |
es |
x–o |
0.4 |
– |
– |
197 |
Gomphonema micropus Kützing |
B |
– |
str |
ind |
i |
es |
o |
1.3 |
ot |
ate |
198 |
Gomphonema montanum (Schumann J) Grunow in Schneider |
B |
– |
str |
ind |
i |
es |
x–b |
0.85 |
m |
ats |
199 |
Gomphonema olivaceum (Hornemann) Brébisson var. olivaceum |
B |
– |
st–str |
alf |
i |
es |
o–b |
1.45 |
e |
ate |
200 |
Gomphonema olivaceum var. minutissimum Hustedt |
B |
– |
str |
alf |
i |
– |
o |
1.2 |
o–m |
ats |
201 |
Gomphonema parvulum (Kützing) Kützing var. parvulum |
B |
temp |
str |
ind |
i |
es |
b |
2.35 |
o–m |
hne |
202 |
Gomphonema parvulum var. subellipticum Cleve |
B |
temp |
str |
ind |
i |
es |
b |
2.35 |
o–m |
hne |
203 |
Gomphonema productum (Grunow) Lange–Bertalot & Reichardt in Lange–Bertalot |
B |
– |
str |
ind |
i |
es |
o |
1.3 |
o–m |
ate |
204 |
Gomphonema salinarum (Pantosek) Cleve |
B |
– |
– |
– |
mh |
– |
– |
– |
– |
– |
205 |
Gomphonema subclavatum (Grunow) Grunow |
B |
– |
str |
ind |
i |
es |
o |
1.1 |
o–m |
ats |
206 |
Gomphonema truncatum Ehrenberg |
B |
– |
st–str |
ind |
i |
es |
o–b |
1.4 |
me |
ats |
207 |
Gomphonema ventricosum Gregory |
B |
cool |
str |
ind |
i |
– |
x |
0.3 |
ot |
ats |
208 |
Gomphonema vibrio var. pumilum (Grunow) R.Ross in B.Hartley, Ross R & Williams DM |
B |
– |
– |
– |
– |
sp |
– |
– |
– |
– |
209 |
Gyrosigma acuminatum (Kützing) Rabenhorst |
B |
cool |
st–str |
alf |
i |
es |
o–a |
1.95 |
me |
ate |
210 |
Gyrosigma attenuatum (Kützing) Rabenhorst |
P–B |
– |
st |
alf |
i |
– |
o–a |
1.8 |
o–m |
ate |
211 |
Gyrosigma obtusatum (Sullivant & Wormley) Boyer CS |
B |
– |
str |
alf |
i |
sp |
b |
2 |
e |
ate |
212 |
Gyrosigma peisone (Grunow) Hustedt in Pascher |
B |
– |
st–str |
alf |
mh |
es |
o |
1 |
me |
– |
213 |
Gyrosigma scalproides (Rabenhorst) Cleve |
B |
– |
– |
alf |
i |
es |
b |
2.2 |
– |
– |
214 |
Halamphora acutiuscula (Kützing) Levkov |
P–B |
warm |
– |
alf |
mh |
sp |
b |
2 |
– |
– |
215 |
Halamphora coffeaeformis (Agardh) Levkov |
B |
– |
st–str |
alf |
mh |
– |
a |
3 |
e |
ate |
216 |
Halamphora normanii (Rabenhorst) Levkov |
B |
– |
ae |
alf |
hb |
– |
x |
0.1 |
m |
ats |
217 |
Halamphora perpusilla (Grunow) Q.– You M & Kociolek JP in You et al. |
B |
– |
– |
alf |
mh |
– |
– |
– |
– |
– |
218 |
Halamphora subcapitata (Kisselew) Levkov |
B |
– |
– |
– |
hl |
– |
o |
1 |
– |
– |
219 |
Halamphora veneta (Kützing) Levkov |
B |
– |
st–str |
alf |
i |
es |
a–o |
2.6 |
e |
ate |
220 |
Hannaea arcus (Ehrenberg) Patrick emend. Genkal et Kharitonov var. arcus |
B |
temp |
str |
alf |
i |
es |
x |
0.3 |
o–m |
ats |
221 |
Hannaea arcus var. amphioxys (Rabenhorst) Patrick RM |
B |
cool |
str |
alf |
i |
– |
x |
0.3 |
– |
– |
222 |
Hannaea linearis (Holmboe) Álvarez–Blanco & Blanco S |
P |
– |
– |
alf |
i |
– |
– |
– |
– |
– |
223 |
Hantzschia amphioxys (Ehr.) Grunow var. amphioxys |
B |
temp |
st–str |
ind |
I |
es |
o–a |
1.9 |
o–e |
ate |
224 |
Hantzschia amphioxys var. vivax (Hantzsch) Grunow in Cleve & Grunow |
B |
– |
– |
alb |
hl |
– |
o–a |
1.9 |
– |
– |
225 |
Hantzschia compacta (Hustedt) Lange–Bertalot in Lange–Bertalot & Genkal |
B |
– |
– |
– |
i |
– |
b |
2 |
– |
– |
226 |
Hippodonta capitata (Ehrenberg) Lange–Bertalot, Metzeltin & Witkowski |
B |
temp |
st–str |
alf |
hl |
es |
b |
2.1 |
me |
ate |
227 |
Hippodonta hungaricа (Grunow) Lange–Bertalot, Metzeltin and Witkowski |
B |
– |
st–str |
alf |
hl |
es |
b |
2.3 |
me |
ate |
228 |
Humidophila contenta (Grunow) Lowe, Kociolek, Johansen JR, Van de Vijver, Lange–Bertalot & Kopalová |
B, aer |
– |
str |
ind |
i |
es |
o–x |
0.7 |
o–m |
ate |
229 |
Humidophila perpusilla (Grunow) Lowe, Kociolek, Johansen, Van de Vijver, Lange–Bertalot & Kopalová |
B |
warm |
str |
ind |
i |
sp |
o–x |
0.7 |
o–m |
ats |
230 |
Iconella helvetica (Brun) Ruck & Nakov in Ruck et al. |
B |
– |
– |
ind |
– |
– |
b |
2 |
ot |
– |
231 |
Iconella linearis (Smith W) Ruck & Nakov in Ruck et al. |
P–B |
– |
– |
ind |
i |
es |
x–o |
0.5 |
o–m |
– |
232 |
Iconella tenera (Gregory W) Ruck & Nakov in Ruck et al. |
P–B |
– |
st |
alf |
i |
es |
o |
1.1 |
ot |
– |
233 |
Kobayasiella micropunctata (Germain) Lange–Bertalot |
B |
– |
– |
acb |
hb |
– |
x |
0.3 |
– |
ats |
234 |
Kobayasiella subtilissima (Cleve) Lange–Bertalot |
B,S |
– |
st |
– |
– |
– |
b |
2 |
– |
– |
235 |
Kurtkrammeria aequalis (Smith W) Bahls L |
P–B |
– |
– |
ind |
i |
– |
o |
1 |
ot |
– |
236 |
Lacustriella lacustris (Gregory W) Lange–Bertalot & Kulikovskiy MS in Kulikovskiy et al. |
B |
– |
– |
ind |
i |
– |
o |
1 |
o–m |
ats |
237 |
Lindavia antiqua (Smith W) Nakov et al. |
P–B |
– |
– |
acf |
hb |
– |
o |
1 |
ot |
ats |
238 |
Lindavia bodanica (Eulenstein ex Grunow) Nakov T et al. |
P |
– |
st |
ind |
i |
– |
x |
1 |
ot |
ats |
239 |
Lindavia comta (Kützing) Nakov T et al. |
P |
– |
st |
alf |
i |
sx |
o |
1.2 |
o–m |
– |
240 |
Lindavia kuetzingiana (Thwaites) Nakov T et al. var. kuetzingiana |
P–B |
temp |
st |
ind |
I |
sp |
b |
2.1 |
o–m |
– |
241 |
Lindavia kuetzingiana var. radiosa (Fricke) Nakov T et al. |
P–B |
temp |
st |
ind |
hl |
sp |
b |
2.2 |
– |
– |
242 |
Lindavia schumannii (Grunow) Nakov T et al. |
P |
– |
– |
ind |
hl |
– |
– |
– |
– |
– |
243 |
Luticola cohnii (Hilse) Mann in Round DG, Crawford RM & Mann DG |
B, aer |
– |
ae |
ind |
i |
es |
o |
1 |
e |
ate |
244 |
Luticola mutica (Kützing) Mann DG in Round et al. |
B,S |
– |
st–str |
ind |
i |
sp |
o–a |
1.9 |
e |
ate |
245 |
Luticola muticopsis (Van Heurck) Mann in Round DG, Crawford RM & Mann DG |
B |
– |
st–str |
– |
– |
– |
– |
– |
– |
– |
246 |
Luticola nivalis (Ehrenberg) Mann in Round DG, Crawford RM & Mann DG |
B,S |
– |
ae |
ind |
hl |
– |
o–a |
1.9 |
e |
– |
247 |
Mastogloia baltica Grunow in Van Heurck |
B |
– |
– |
– |
mh |
– |
– |
– |
– |
– |
248 |
Mastogloia braunii Grunow |
P–B |
– |
– |
alf |
mh |
– |
– |
– |
– |
– |
249 |
Mastogloia elliptica (Agardh C) Cleve in Schmidt et al. |
B |
– |
– |
alf |
mh |
– |
– |
– |
– |
– |
250 |
Mastogloia lacustris (Grunow) Grunow in Van Heurck |
B |
– |
str |
alf |
hl |
– |
o |
1.3 |
e |
ats |
251 |
Mastogloia pumila (Grunow) Cleve |
B |
– |
– |
– |
mh |
– |
– |
– |
– |
– |
252 |
Mastogloia smithii Thwaites |
B |
– |
– |
alf |
mh |
sx |
o |
1.3 |
me |
– |
253 |
Meridion circulare (Greville) Agardh |
B |
– |
str |
ind |
i |
es |
o |
1.1 |
o–m |
ate |
254 |
Meridion constrictum Ralfs |
P–B |
– |
st–str |
ind |
hb |
sx |
o |
1.1 |
o–e |
ate |
255 |
Navicula bryophila Østrup |
B |
– |
str |
neu |
hb |
es |
o–x |
0.7 |
m |
ats |
256 |
Navicula capitatoradiata H.Germain |
P–B |
– |
st–str |
alf |
mh |
sx |
b |
2.1 |
me |
ate |
257 |
Navicula cari Ehrenberg |
P–B |
– |
str |
ind |
i |
es |
b–a |
2.4 |
o–m |
ats |
258 |
Navicula cincta (Ehrenberg) Ralfs |
B |
warm |
st–str |
alf |
hl |
es |
x–o |
0.5 |
me |
ate |
259 |
Navicula cryptocephala Kützing var. cryptocephala |
P–B |
temp |
st–str |
ind |
i |
es |
b |
2.1 |
o–e |
ate |
260 |
Navicula cryptocephala var. lata Poretz. et Anissimova |
B |
– |
– |
– |
i |
– |
– |
– |
– |
– |
261 |
Navicula dicephala Ehrenberg |
B |
– |
– |
ind |
i |
– |
o–b |
1.4 |
– |
– |
262 |
Navicula digitoradiatа (Gregory) Ralfs in Prichard |
B |
– |
– |
alf |
I |
es |
b |
2 |
me |
– |
263 |
Navicula exilis Kützing |
B |
– |
– |
alb |
hl |
es |
x–b |
0.8 |
ot |
– |
264 |
Navicula gothlandica Grunow in Van Heurck |
P–B |
– |
– |
alf |
hl |
es |
b |
2 |
o–m |
– |
265 |
Navicula gregaria Donkin |
P–B |
– |
– |
alf |
I |
es |
b–a |
2.5 |
me |
ate |
266 |
Navicula halophila f. subcapitata (Østrup) Krasske |
B |
– |
st–str |
alf |
mh |
es |
– |
– |
– |
– |
267 |
Navicula hofmanniae Lange–Bertalot |
B |
– |
– |
alf |
oh |
– |
o |
1 |
– |
– |
268 |
Navicula lacustris var. parallela Wisl. & Kolbe |
B |
– |
– |
ind |
i |
– |
– |
– |
– |
– |
269 |
Navicula lacustris var. paulseniana (Petersen JB) Zabelina |
B |
– |
– |
– |
i |
– |
– |
– |
– |
– |
270 |
Navicula libonensis Schoeman |
P–B |
– |
– |
alf |
I |
– |
b–a |
2.4 |
o–m |
– |
271 |
Navicula lucidula Grunow in Van Heurck |
B |
– |
– |
– |
i |
– |
– |
– |
– |
– |
272 |
Navicula menisculus Schumann |
P–B |
– |
st–str |
alf |
i |
es |
o–b |
1.45 |
o–m |
ate |
273 |
Navicula minima Grunow in Van Heurck |
P–B |
– |
– |
alf |
hl |
es |
a–o |
2.6 |
e |
hne |
274 |
Navicula peregrina (Ehrenberg) Kützing |
P–B |
– |
– |
alf |
mh |
es |
o–b |
1.5 |
o–m |
– |
275 |
Navicula radiosa Kützing |
B |
temp |
st–str |
ind |
i |
es |
o |
1.3 |
me |
ate |
276 |
Navicula recens (Lange–Bertalot) Lange–Bertalot in Krammer & Lange–Bertalot |
P–B |
– |
– |
alf |
i |
es |
b–a |
2.5 |
e |
– |
277 |
Navicula rhynchocephala Ehrenberg |
B |
– |
– |
alf |
hl |
– |
o–a |
1.95 |
o–m |
ate |
278 |
Navicula rostellata Kützing |
B |
– |
st–str |
alf |
i |
es |
b |
2.2 |
e |
ate |
279 |
Navicula rotaeana (Rabenhorst) Grunow in Van Heurck |
P–B |
– |
st |
ind |
i |
– |
o–x |
0.7 |
ot |
– |
280 |
Navicula salinarum f. minima Kolbe |
P–B |
– |
st–str |
ind |
mh |
– |
b |
2.1 |
me |
ate |
281 |
Navicula semen Ehrenberg |
B |
– |
– |
ind |
i |
– |
o |
1 |
ot |
– |
282 |
Navicula tripunctata (Müller OF) Bory in Bory de Saint–Vincent |
P–B |
– |
st–str |
ind |
i |
es |
b–o |
1.7 |
e |
ate |
283 |
Navicula veneta Kützing |
B |
– |
– |
alf |
hl |
es |
a–o |
2.7 |
– |
– |
284 |
Navicula viridula (Kützing) Ehrenberg |
B |
– |
st–str |
alf |
hl |
es |
b |
2.2 |
me |
ate |
285 |
Navicula vulpina Kützing |
B |
– |
str |
ind |
i |
– |
b |
2 |
me |
ats |
286 |
Navicymbula pusilla (Grunow in Schmidt A) Krammer |
B |
– |
– |
alf |
mh |
es |
– |
– |
– |
– |
287 |
Neidiomorpha binodis (Ehrenberg) M.Cantonati, Lange–Bertalot & Angeli N |
B |
– |
str |
alf |
i |
– |
o |
1 |
me |
ate |
288 |
Neidium affine (Ehrenberg) Pfizer var. affine |
B |
– |
str |
ind |
i |
– |
o–x |
0.7 |
ot |
ats |
289 |
Neidium affine var. amphirhynchus (Ehrenberg) Cleve |
B |
– |
– |
alb |
hb |
– |
o–x |
0.7 |
– |
– |
290 |
Neidium iridis (Ehrenberg) Cleve |
B |
– |
st–str |
ind |
hb |
es |
o–x |
0.6 |
ot |
ats |
291 |
Neidium kozlowii Mereschkovsky |
B |
– |
– |
ind |
i |
– |
o |
1 |
ot |
– |
292 |
Neidium productum (Smith W) Cleve |
P–B |
temp |
– |
ind |
i |
sx |
x–b |
0.9 |
ot |
ats |
293 |
Neidium punctulatum Hustedt |
B |
– |
– |
ind |
i |
– |
– |
– |
– |
– |
294 |
Nitzschia amphibia Grunow var. amphibia |
P–B, S |
temp |
st–str |
alf |
i |
sp |
b |
2.1 |
e |
hne |
295 |
Nitzschia angustata var. acuta Grunow in Cleve & Grunow |
B |
– |
– |
alf |
i |
– |
b |
2 |
– |
– |
296 |
Nitzschia brevissima Grunow in Van Heurck |
– |
– |
st–str |
alf |
hl |
es |
o–a |
1.9 |
e |
– |
297 |
Nitzschia communis Rabenhorst |
P–B |
– |
st–str |
ind |
i |
sp |
a |
3.1 |
e |
hce |
298 |
Nitzschia dissipata (Kützing) Rabenhorst |
B |
– |
st–str |
alf |
i |
sx |
b–o |
1.7 |
me |
ate |
299 |
Nitzschia dubia Smith W |
P–B |
– |
st–str |
alf |
I |
– |
a–o |
2.7 |
e |
hne |
300 |
Nitzschia fasciculata (Grunow) Grunow in Van Heurck |
B |
– |
st |
alf |
hl |
sx |
o |
1.1 |
– |
– |
301 |
Nitzschia fonticola (Grunow) Grunow in Van Heurck |
P–B |
– |
st–str |
alf |
I |
– |
o–b |
1.5 |
me |
ate |
302 |
Nitzschia frustulum (Kützing) Grunow in Cleve & Grunow var. frustulum |
P–B |
temp |
st–str |
alf |
I |
sp |
b |
2.3 |
e |
hce |
303 |
Nitzschia frustulum var. subsalina Hustedt |
B |
– |
– |
alb |
hl |
sp |
b |
2 |
– |
– |
304 |
Nitzschia frustulum var. perpusilla (Rabenhorst) Van Heurck |
B |
– |
– |
alf |
hl |
es |
a–o |
2.7 |
– |
– |
305 |
Nitzschia gracilis Hantzsch |
P–B |
temp |
st–str |
ind |
i |
sp |
o–a |
1.8 |
m |
– |
306 |
Nitzschia gradifera Hustedt |
B |
– |
– |
– |
hl |
– |
– |
– |
– |
– |
307 |
Nitzschia hantzschiana Rabenhorst |
P–B |
– |
str |
alf |
i |
es |
x–o |
0.5 |
m |
ats |
308 |
Nitzschia holsatica Hustedt |
P–B |
– |
– |
ind |
i |
es |
b |
2.3 |
– |
– |
309 |
Nitzschia kittlii Grunow |
– |
– |
– |
– |
hl |
– |
– |
– |
– |
– |
310 |
Nitzschia kuetzingiana Hilse |
B |
– |
– |
ind |
hl |
es |
b |
2.1 |
– |
– |
311 |
Nitzschia linearis var. tenuis (Smith W) Grunow in Cleve & Grunow |
B |
– |
str |
alf |
i |
es |
b–o |
1.7 |
me |
– |
312 |
Nitzschia linearis W.Smith var. linearis |
B |
temp |
st–str |
alf |
i |
es |
b–o |
1.7 |
me |
ate |
313 |
Nitzschia microcephala Grunow in Cleve & Möller |
P–B |
– |
st–str |
alf |
I |
sx |
b |
2.3 |
e |
hce |
314 |
Nitzschia obtusa Smith W |
B |
– |
– |
– |
hl |
es |
b–a |
2.4 |
m |
– |
315 |
Nitzschia ostenfeldii Hustedt |
B |
– |
– |
– |
i |
– |
– |
– |
– |
– |
316 |
Nitzschia palea (Kützing) Smith W |
P–B |
temp |
– |
ind |
i |
sp |
a–o |
2.8 |
he |
hce |
317 |
Nitzschia paleacea Grunow in Van Heurck |
P–B |
– |
st–str |
alf |
i |
es |
b |
2.2 |
e |
hce |
318 |
Nitzschia pusilla Grunow 1862 |
P–B, S |
– |
st–str |
alf |
i |
es |
b–o |
1.7 |
o–e |
ate |
319 |
Nitzschia regula Hustedt |
– |
– |
– |
– |
– |
– |
o |
1 |
– |
– |
320 |
Nitzschia sigma (Kützing) Smith var W. sigma |
B |
temp |
st–str |
alf |
mh |
es |
a |
3 |
e |
ate |
321 |
Nitzschia sigma var. curvula (Ehrenberg) Brun. |
B |
– |
– |
– |
mh |
– |
– |
– |
– |
– |
322 |
Nitzschia sigmoidea (Nitzsch) Smith W |
P–B |
– |
st–str |
alf |
i |
– |
b–a |
2.5 |
e |
ate |
323 |
Nitzschia sublinearis Hustedt |
P–B |
– |
– |
alf |
i |
es |
a |
3 |
me |
– |
324 |
Nitzschia supralitorea Lange–Bertalot |
B |
– |
st–str |
alf |
oh |
sp |
o–b |
1.5 |
e |
hne |
325 |
Nitzschia thermalis (Ehrenberg) Auerswald in Rabenhorst var. thermalis |
P |
– |
– |
ind |
i |
es |
a–o |
2.8 |
– |
– |
326 |
Nitzschia thermalis var. minor Hilse |
B |
– |
st–str |
acf |
– |
– |
o |
1 |
– |
– |
327 |
Nitzschia tryblionella Hantzsch in Rabenhorst |
B |
– |
st–str |
alf |
hl |
– |
a–o |
2.6 |
me |
ate |
328 |
Nitzschia vermicularis (Kützing) Hantzsch in Rabenhorst |
P–B |
– |
str |
alf |
i |
– |
b |
2.2 |
m |
– |
329 |
Nitzschia commutata Grunow in Cleve & Grunow |
P–B |
– |
– |
alf |
mh |
– |
b |
2 |
– |
– |
330 |
Odontidium mesodon (Kützing) Kützing |
B |
cool |
st–str |
neu |
hb |
sx |
x–o |
0.4 |
ot |
ats |
331 |
Odontidium anceps (Ehrenberg) Ralfs |
P–B |
cool |
st–str |
neu |
hb |
sx |
o–x |
0.6 |
ot |
– |
332 |
Odontidium hyemale (Roth) Kützing |
P–B |
cool |
st–str |
ind |
hb |
sx |
x |
0.3 |
ot |
ats |
333 |
Paraplaconeis placentula (Ehrenberg) Kulikovskiy MS & Lange–Bertalot in Kulikowskiy et al. |
B |
temp |
st–str |
alf |
i |
sx |
o–b |
1.5 |
e |
ate |
334 |
Paraplaconeis subplacentula (Hustedt) Kulikovskiy & Lange–Bertalot in Kulikovskiy et al. |
B |
– |
– |
– |
i |
– |
– |
– |
– |
– |
335 |
Parlibellus crucicula (Smith W) Witkowski, Lange–Bertalot & Metzeltin |
B |
– |
– |
ind |
mh |
– |
b |
2 |
– |
– |
336 |
Parlibellus protracta (Grunow) Witkowski, Lange–Bertalot & Metzeltin |
B |
– |
st–str |
ind |
mh |
es |
b–o |
1.7 |
e |
ate |
337 |
Pinnularia abaujensis var. linearis (Hustedt) Patrick RM |
B |
– |
– |
acf |
– |
– |
– |
– |
– |
– |
338 |
Pinnularia acrosphaeria Smith W |
B |
warm |
st |
ind |
i |
es |
x–o |
0.4 |
o–m |
– |
339 |
Pinnularia appendiculata (Agardh C) Schaarschmidt |
B |
– |
str |
ind |
i |
es |
x |
0.3 |
o–m |
ats |
340 |
Pinnularia biceps Gregory W |
B |
– |
str |
acf |
i |
sp |
x–b |
0.8 |
o–m |
ats |
341 |
Pinnularia borealis Ehrenberg |
B |
– |
ae |
ind |
i |
es |
x–o |
0.4 |
o–m |
ate |
342 |
Pinnularia brauniana (Grunow) Studnicka |
P–B |
– |
– |
acf |
i |
– |
x |
0.2 |
ot |
– |
343 |
Pinnularia brevicostata Cleve |
P–B |
cool |
– |
ind |
i |
– |
o |
1 |
ot |
– |
344 |
Pinnularia divergens Smith W |
B |
– |
st |
ind |
i |
– |
x–b |
0.9 |
ot |
– |
345 |
Pinnularia divergentissima (Grunow) Cleve |
P–B |
– |
– |
ind |
i |
– |
o |
1 |
ot |
– |
346 |
Pinnularia elegans (Smith W) Krammer K |
B |
– |
– |
alf |
hl |
– |
b |
2 |
m |
– |
347 |
Pinnularia fonticola Hustedt |
B |
– |
– |
ind |
i |
– |
– |
– |
– |
– |
348 |
Pinnularia gibbiformis Krammer K |
B |
– |
– |
– |
– |
– |
x |
0.3 |
– |
– |
349 |
Pinnularia globiceps Gregory W |
B |
– |
– |
acf |
i |
– |
x |
0.2 |
o–m |
– |
350 |
Pinnularia gracillima Gregory W |
B |
– |
– |
acf |
i |
– |
x |
0.2 |
o–m |
– |
351 |
Pinnularia interrupta Smith W |
B |
– |
– |
ind |
i |
– |
– |
– |
– |
– |
352 |
Pinnularia lata (Brébisson) Smith var W lata |
P–B |
– |
str |
acf |
i |
– |
o |
1 |
ot |
– |
353 |
Pinnularia lata var. minor (Grunow) Cleve |
B |
– |
– |
acf |
hb |
– |
o |
1 |
ot |
– |
354 |
Pinnularia major (Kützing) Rabenhorst |
B |
temp |
st–str |
ind |
i |
– |
o–x |
0.6 |
me |
ate |
355 |
Pinnularia mesolepta (Ehrenberg) Smith W |
P–B |
– |
st–str |
ind |
i |
– |
x–b |
0.8 |
me |
ate |
356 |
Pinnularia microstauron (Ehrenberg) Cleve |
P–B |
temp |
st–str |
ind |
i |
sp |
o–x |
0.7 |
ot |
ate |
357 |
Pinnularia rhombarea var. biundulata (Otto Müller) Krammer |
B |
– |
st–str |
ind |
i |
– |
o |
1 |
ot |
– |
358 |
Pinnularia schoenfelderi Krammer |
B |
– |
– |
ind |
i |
– |
o |
1 |
o–m |
– |
359 |
Pinnularia septentrionalis Krammer K |
B |
– |
– |
ind |
i |
– |
– |
– |
– |
– |
360 |
Pinnularia stomatophora Hustedt |
B |
– |
str |
acf |
i |
– |
x |
0.2 |
ot |
ats |
361 |
Pinnularia subborealis Hustedt |
B |
– |
– |
– |
I |
– |
o |
1 |
ot |
– |
362 |
Pinnularia subcapitata Gregory W1856 |
B |
st–str |
ind |
i |
sp |
o–x |
0.6 |
o–m |
ate |
|
363 |
Pinnularia tabellaria Ehrenberg var. tabellaria |
B |
– |
– |
neu |
hb |
– |
o |
1 |
– |
– |
364 |
Pinnularia viridiformis Krammer |
B |
– |
– |
– |
– |
– |
o–x |
0.7 |
– |
– |
365 |
Pinnularia viridis (Nitzsch) Ehrenberg |
P–B |
temp |
st–str |
ind |
i |
es |
x |
0.3 |
o–e |
ate |
366 |
Placoneis amphibola (Cleve) CoxEJ |
B |
cool |
str |
ind |
i |
– |
o |
1 |
o–m |
ats |
367 |
Placoneis exigua (Gregory) Mereschkovsky |
B |
– |
– |
ind |
i |
es |
o–b |
1.4 |
o–m |
– |
368 |
Placoneis gastrum (Ehrenberg) Mereschkowsky |
B |
– |
st–str |
ind |
i |
sx |
o–b |
1.4 |
e |
ate |
369 |
Planothidium dispar (Cleve) Witkowski, Lange–Bertalot & Metzeltin |
B |
– |
– |
alf |
hl |
– |
b |
2 |
o–e |
– |
370 |
Planothidium lanceolatum (Brébisson ex Kützing) Lange–Bertalot |
P–B |
warm |
st–str |
alf |
i |
sx |
b–o |
1.6 |
e |
ate |
371 |
Planothidium rostratum (Østrup) Lange–Bertalot |
B |
– |
str |
alf |
i |
es |
b–o |
1.6 |
e |
ate |
372 |
Platessa salinarum (Grunow) Lange–Bertalot |
P–B |
– |
st–str |
ind |
mh |
– |
b |
2.1 |
me |
ate |
373 |
Prestauroneis integra (W. Smith) Bruder |
B |
– |
st–str |
neu |
mh |
– |
x–o |
0.4 |
e |
ate |
374 |
Pseudostaurosira brevistriata (Grunow) Williams and Round var. brevistriata |
P–B |
– |
st–str |
alf |
i |
– |
o |
1.2 |
ot |
– |
375 |
Pseudostaurosira brevistriata var. inflata (Pantocsek) M.B.Edlund |
B |
– |
str |
alf |
i |
es |
o |
1.2 |
o–e |
ats |
376 |
Pseudostaurosira elliptica (Schumann) Edlund, Morales & Spaulding |
B |
– |
str |
alf |
i |
– |
b–a |
2.4 |
me |
ats |
377 |
Pseudostaurosira parasitica (W.Smith) Morales |
P–B |
– |
str |
alf |
i |
es |
o–a |
1.9 |
me |
ats |
378 |
Pseudostaurosira subsalina (Hustedt) E.A. Morales |
P–B |
– |
st |
alf |
hl |
es |
o |
1.3 |
me |
ate |
379 |
Punctastriata lancettula (Schumann) Hamilton PB & Siver PA |
B |
cool |
– |
alb |
i |
es |
b–a |
2.4 |
– |
– |
380 |
Reimeria sinuata (Greg.) Kociolek et Stoermer |
P–B, aer |
– |
st |
ind |
i |
sx |
o |
1.3 |
m |
ate |
381 |
Rhoicosphenia abbreviata (Agardh) Lange–Bertalot |
B |
– |
st–str |
alf |
i |
es |
o–a |
1.9 |
me |
ate |
382 |
Rhopalodia gibba (Ehrenberg) Otto Müller var. gibba |
B |
temp |
– |
alf |
i |
es |
o–b |
1.4 |
o–m |
– |
383 |
Rhopalodia gibba var. ventricosa (Kützing) Mayer |
B |
temp |
– |
alf |
i |
es |
o–b |
1.4 |
– |
– |
384 |
Rhopalodia gibberula (Ehrenberg) O Müller var. gibberula |
B |
temp |
str |
alf |
mh |
es |
b |
2 |
me |
– |
385 |
Rhopalodia gibberula var. producta (Grunow) Otto Müller |
B |
– |
str |
alf |
hl |
– |
– |
– |
– |
– |
386 |
Rhopalodia musculus (Kützing) O Müller var. musculus |
P–B, S |
– |
str |
alb |
mh |
– |
o |
1 |
– |
– |
387 |
Rhopalodia musculus var. mirabilis Fricke |
B |
– |
– |
alf |
hl |
– |
b |
2 |
me |
– |
388 |
Sellaphora bacillum (Ehrenberg) DG Mann |
B |
– |
st–str |
alf |
i |
sx |
o–b |
1.5 |
me |
ats |
389 |
Sellaphora pupula (Kützing) Mereschkowsky |
B |
eterm |
st |
ind |
hl |
sx |
o–a |
1.9 |
me |
ate |
390 |
Sellaphora rectangularis (Gregory W) Lange–Bertalot & Metzeltin |
B |
temp |
st–str |
ind |
hl |
sx |
o–a |
1.9 |
me |
ate |
391 |
Sellaphora tridentula (Krasske) Wetzel CE in Wetzel et al. |
B |
– |
– |
acf |
i |
es |
o |
1 |
ot |
– |
392 |
Stauroneis acuta Smith W |
B |
– |
st–str |
alf |
i |
– |
o |
1 |
o–m |
– |
393 |
Stauroneis anceps Ehrenberg var. anceps |
P–B |
– |
st–str |
ind |
i |
sx |
o |
1.3 |
o–m |
ate |
394 |
Stauroneis borrichii (Petersen JB) Lund JWG |
B |
– |
ae |
neu |
hb |
– |
o |
1 |
– |
– |
395 |
Stauroneis gracilis Ehrenberg |
B |
– |
– |
ind |
I |
– |
o–x |
0.7 |
o–m |
– |
396 |
Stauroneis phoenicenteron (Nitzsch) Ehrenberg |
P–B |
temp |
st–str |
ind |
i |
es |
o |
1.3 |
me |
ate |
397 |
Stauroneis smithii Grunow |
P–B |
– |
st–str |
alf |
i |
– |
o–b |
1.5 |
o–e |
ate |
398 |
Staurosira binodis (Ehrenberg) Lange–Bertalot |
P–B |
– |
str |
alf |
i |
es |
o |
1.3 |
me |
ate |
399 |
Staurosira construens Ehrenberg var. construens |
P–B |
temp |
st–str |
alf |
i |
sx |
o |
1.3 |
me |
ats |
400 |
Staurosira construens var. exigua (Smith W) Nagumo T |
B |
– |
str |
alf |
i |
sp |
o |
1.3 |
me |
ate |
401 |
Staurosira pseudoconstruens (Marciniak) Lange–Bertalot |
– |
– |
– |
– |
i |
– |
o |
1 |
ot |
– |
402 |
Staurosira venter (Ehrenberg) Cleve & JD Möller |
P–B |
warm |
st–str |
alf |
i |
sx |
o |
1.3 |
me |
ate |
403 |
Staurosirella harrisonii (W.Smith) E. Morales & Wetzel CE in Morales et al. |
B |
– |
st |
alf |
hb |
es |
a–o |
2.7 |
– |
– |
404 |
Staurosirella leptostauron (Ehrenberg) Williams and Round |
P–B |
– |
st |
alf |
hb |
es |
o |
1.1 |
me |
ats |
405 |
Staurosirella pinnata (Ehrenberg) Williams and Round |
P–B |
temp |
st–str |
alf |
hl |
es |
o |
1.2 |
o–e |
ate |
406 |
Stephanodiscus astraea (Ehrenberg) Grunow in Cleve & Grunow var. astraea |
P |
temp |
st |
alb |
i |
es |
o–b |
1.5 |
me |
– |
407 |
Stephanodiscus astraea var. intermedia Fricke |
P |
– |
– |
ind |
i |
– |
– |
– |
– |
– |
408 |
Stephanodiscus minutulus (Kützing) Cleve & Möller 1882 |
P |
temp |
st |
alb |
i |
es |
b |
2.2 |
o–m |
ate |
409 |
Surirella angusta Kützing |
P–B |
– |
st–str |
alf |
i |
es |
b–o |
1.7 |
e |
ate |
410 |
Surirella angustata Hustedt var. angustata |
P–B |
– |
st–str |
alf |
i |
– |
b–o |
1.7 |
e |
– |
411 |
Surirella angustata var. constricta Hustedt |
B |
– |
– |
– |
i |
– |
– |
– |
– |
– |
412 |
Surirella brebissonii Krammer and Lange–Bertalot |
B |
– |
st–str |
alf |
i |
– |
b–o |
1.7 |
– |
– |
413 |
Surirella minuta Brébisson in Kützing |
B |
– |
st–str |
ind |
i |
es |
o–a |
1.8 |
me |
– |
414 |
Surirella ovalis Brébisson |
P–B |
– |
st–str |
alf |
I |
es |
a |
3 |
me |
ate |
415 |
Surirella ovata var. crumena (Brébisson) Hustedt |
B |
– |
– |
alf |
hl |
– |
o–a |
1.9 |
– |
– |
416 |
Surirella spiralis Kützing |
B |
– |
str |
alf |
i |
– |
x |
0.2 |
ot |
ats |
417 |
Surirella splendida (Ehrenberg) Kützing |
P–B |
– |
st–str |
alf |
i |
– |
o–x |
0.7 |
me |
– |
418 |
Synedra nana Meister F |
– |
– |
– |
ind |
hb |
es |
o |
1 |
– |
– |
419 |
Synedra spectabilis Ehrenberg |
B |
– |
– |
alf |
I |
– |
b–a |
2.5 |
m |
– |
420 |
Tabellaria fenestrata (Lyngbye) Kützing |
P–B |
– |
st–str |
ind |
i |
es |
x |
0.3 |
o–m |
ats |
421 |
Tabellaria flocculosa (Roth) Kützing |
P–B |
eterm |
st–str |
acf |
i |
es |
o–x |
0.6 |
ot |
ats |
422 |
Tabularia fasciculata (Agardh C) Williams DM & Round |
P–B |
– |
st |
ind |
mh |
es |
b–a |
2.5 |
e |
ate |
423 |
Tetracyclus rupestris (Kützing) Grunow in Van Heurck |
P–B |
cool |
ae |
acf |
i |
– |
x–b |
0.8 |
ot |
– |
424 |
Tryblionella angustata Smith W |
P–B |
– |
st |
alf |
i |
sx |
o–b |
1.5 |
m |
ats |
425 |
Tryblionella apiculata W.Gregory |
B |
– |
– |
alf |
hl |
es |
a–o |
2.7 |
e |
– |
426 |
Tryblionella hungarica (Grunow) Frenguelli |
P–B |
– |
– |
alf |
mh |
sp |
a–o |
2.9 |
e |
ate |
427 |
Tryblionella levidensis W.Smith |
P–B |
– |
st–str |
ind |
mh |
sp |
a–o |
2.6 |
e |
ate |
428 |
Tryblionella victoriae Grunow |
B |
– |
st–str |
alf |
hl |
sp |
a–o |
2.6 |
e |
ate |
429 |
Ulnaria acus Aboal |
P–B |
– |
st–str |
alf |
i |
es |
o–a |
1.85 |
o–m |
– |
430 |
Ulnaria amphirhynchus (Ehrenberg) Compère & Bukhtiyarova |
P–B |
– |
– |
alf |
i |
es |
b |
2 |
o–m |
– |
431 |
Ulnaria biceps (Kützing) Compére |
P–B |
temp |
– |
alf |
i |
– |
o–a |
1.9 |
o–m |
– |
432 |
Ulnaria capitata (Ehrenberg) Compère |
P–B |
– |
st–str |
alf |
i |
es |
o–b |
1.5 |
e |
ats |
433 |
Ulnaria contracta (Østrup) Morales EA & Vis ML |
B |
– |
– |
alf |
i |
es |
b–a |
2.4 |
– |
– |
434 |
Ulnaria danica (Kützing) Compère & Bukhtiyarova in Bukhtiyarova & Compère |
P–B |
temp |
– |
alf |
i |
es |
b–o |
1.7 |
o–m |
– |
435 |
Ulnaria delicatissima var. angustissima (Grun.) Aboal et Silva |
P |
– |
– |
alf |
i |
es |
b–o |
1.7 |
o–m |
– |
436 |
Ulnaria oxyrhynchus (Kützing) Aboal in Aboal, Alvarez Cobelas, Cambra & Ector |
P–B |
– |
– |
alf |
i |
es |
b–a |
2.4 |
o–m |
– |
437 |
Ulnaria ulna (Nitzsch) Compére var. ulna |
P–B |
temp |
st–str |
ind |
i |
es |
b |
2.25 |
o–e |
ate |
438 |
Ulnaria ulna var. aequalis (Kützing) Aboal in Aboal, Alvarez Cobelas, Cambra & Ector |
P–B |
– |
– |
alf |
i |
sp |
b |
2 |
o–m |
– |
Table 1 Diversity of diatom species–indicators in the Pamir aquatic habitats with autecological characteristics and species–specific Indices of saprobity. Abbreviations of groups of indicators and the autecology of species are given in the note to the table.
Note: Substrate preferences (Hab): P – planktonic, P–B – plankto–benthic, B – benthic, S – soil, Ep – epiphyte, ae – aerophyte. Temperature preferences (T): temp – temperate temperature, eterm – eurythermic, warm – warm–water, cool – could water inhabitants. Oxygenation and streaming (Oxy): st – standing water, str – streaming water, st–str – low streaming water, ae – aerophiles. Halobity degree (Sal): i – oligohalobes–indifferent, hl – halophiles, hb – halophobes. Acidity degree (pH): alf – alkaliphiles, ind – indifferents; acf – acidophiles, alb – alkalibiontes, neu, neutrophiles, acb – acidobiontes. Organic pollution indicators according to Watanabe (D): sx – saproxenes, es – eurysaprobes, sp – saprophiles. Species–specific index of saprobity S (S). Organic pollution indicators according to Sládeček (Sap): x – xenosaprob, x–o – xeno–oligosaprob, o–x – oligo–xenosaprob, x–b – xeno–betamesosaprob, o – oligosaprob, o–b – oligo–beta–msosaprob, b–o – beta–oligosaprob, o–a – oligo–alpha–mesosaprob, b – beta–mesosaprob, b–a – beta–alpha–mesosaprob, a – alpha–mesosaprob, a–b – alpha–beta–mesosaprob, b–p – beta–polysaprob. Nitrogen uptake metabolism (Aut–Het): ats – nitrogen–autotrophic taxa, tolerating very small concentrations of organically bound nitrogen; ate – nitrogen–autotrophic taxa, tolerating elevated concentrations of organically bound nitrogen; hne – facultatively nitrogen–heterotrophic taxa, needing periodically elevated concentrations of organically bound nitrogen, hce – facultatively nitrogen–heterotrophic taxa, needing elevated concentrations of organically bound nitrogen. Trophic state (Tro): ot – oligotraphentic; o–m – oligo–mesotraphentic; m – mesotraphentic; me – meso–eutraphentic; e – eutraphentic; o–e – oligo–eutraphentic, he – hypereutraphentic. – property is unknown.
Altogether 558 diatom taxa were revealed for total list of diatom algae in Pamir habitats,12 and 438 of them were bioindicators. Twelve environmental properties were assessed with help of species autecology. Bioindication analysis revealed prevalence of species preferring benthic or periphytonic lifestyle (433 indicators), temperate water temperature with significant number of cool–water and warm–water indicators (92 taxa). Pamir diatoms were mostly represented by slow streaming groups but streaming water indicators were also numerous (266 indicators). Water–pH indicators were mostly represented by pH–indifferent group and alkaliphilic species that let us to characterize the Pamir water as low–alkaline (395 indicators). The water salinity as one of important variable for aquatic diversity was indicate the waters in Pamir as fresh and low–saline (416 indicators). The sunlight energy utilization property for diatoms is strongly prevail as show indication of photosynthetic way in nutrition for organisms in aquatic ecosystems of Pamir (208 indicators). The indication of Pamir water bodies trophic state show two different groups of species reflect of oligotrophic and meso–eutrophic properties of studied water bodies (324 indicators). Organic pollution indication according T. Watanabe reflects low–polluted waters in Pamir that usually represent of fresh clear natural waters (246 indicators). The same variable in the Sládeček system with 388 indicator taxa help us to characterize Pamir waters as natural clear or slightly organically polluted, with water quality Class 2 and 3. As results, bioindication of Pamir waters by diatoms show natural slowly streaming low alkaline and low saline fresh waters with low level of organic pollution inhabited with species that preferred photosynthesis for proteins creation. Thereby, bioindication revealed the numerous species that are unique in Pamir diatom flora, about 22%, which have not now any information yet about its ecological properties. The important property of revealed diatom flora can be not only large species richness but also wide amplitude of the environmental variables in water temperature, oxygen saturation, salinity, and trophic state of water bodies which they inhabited. Therefore, this first experience of bioindication implementation to Pamir environment assessment demonstrated not only importance of ecological approach to environmental properties analysis in hard–to–reach high mountain habitats but also large perspectives of the method for the system of nature protection in the face of anthropogenic pollution press and climate change.
The work was partly supported by the Israeli Ministry of Aliyah and Integration.
Author declares there is no conflict of interest.

©2018 Niyatbekov, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.