MOJ
eISSN: 2573-2919


Research Article Volume 9 Issue 6
Departament of Chemical Engineering, Escola Politécnica, Universidade de São Paulo, USP, Brazil
Correspondence: Luiz Kulay, Departament of Chemical Engineering, Escola Politécnica, Universidade de São Paulo, USP, Av. Prof. Lineu Prestes, 580, Zip Code: 05508-000, São Paulo, SP, Brazil, Tel (+55) 11 3091.2233
Received: November 10, 2024 | Published: November 21, 2024
Citation: Valdés SC, Carvalho K, Pacheco KA. Assessment of environmental performance of an alternative acetic acid production route based on CO2 recovery. MOJ Eco Environ Sci. 2024;9(6):241-250. DOI: 10.15406/mojes.2024.09.00332
The chemical industry considers synthesizing chemical products from carbon dioxide a promising, sustainable, and environmentally friendly approach. This process involves replacing fossil fuels with renewable raw materials, reusing waste, and optimizing mass and energy flows within the industry. Following in the same direction, Carbon Capture and Utilization (CCU) is an appealing technology that allows for the valorization of CO2, enabling its use in various processes. However, a more comprehensive assessment beyond the CCU influence is necessary to realize the anticipated benefits – particularly environmental ones. This study investigated the environmental feasibility of using CO2 emitted from stationary sources as a feedstock for acetic acid synthesis. To achieve this, we compared the environmental performance of this alternative route, assessed through the Life Cycle Assessment (LCA) technique, with a conventional method for manufacturing the organic acid. As this is an exploratory assessment, the evaluation focused on estimating impacts regarding Primary Energy Demand (PED) and Global Warming Potential (GWP). The study also discussed the potential implications of utilizing renewable energy for CH3COOH production and examined suggestions for environmental improvements based on Carbon Capture and Utilization. The research findings indicate that the alternative approach could lead to a 64% reduction in PED contributions and a 47% decrease in GWP effects compared to the performance achieved through the conventional method.
Keywords: acetic acid, carbon capture utilization and storage, environmental performance, LCA
CCU, carbon capture and utilization; LCA, life cycle assessment; PED, primary energy demand; GWP, global warming potential; GHG, greenhouse gases; CH3COOH, acetic acid; LCI, life cycle inventory; IPCC, Intergovernmental panel on climate change; CED, cumulative energy demand; LCIA, life cycle impact assessment; ATR, auto-thermal reforming; RWGS, reverse water gas shift; POX, partial oxidation; PSA, pressure swing adsorption; RF, raw feed
Human activities, such as burning fossil fuels, deforestation, and agriculture, increasingly impact the climate system. These actions contribute to global warming and trigger rapid changes unprecedented in recorded history. Greenhouse Gases (GHG) concentrations like CO2, CH4, and N2O in the atmosphere are at their highest levels in 800,000 years. Without urgent measures to curb these emissions, global warming could exceed an average increase of 1.5°C before mid-century. In this context, decarbonizing industrial processes is crucial for effective climate action. The Carbon Capture and Utilization (CCU) process can offer a low-carbon pathway for the chemical industry, providing an opportunity to enhance climate compliance.1
Over the years, significant progress has been made in utilizing CO2 as a raw material to synthesize products from chemical and petrochemical industries. Innovative solvents and solutions have been developed to address the low energy capacity of such reactions and make them feasible. Additionally, renewable energy sources have been systematically integrated into these processes. Jarvis and Samsatli2 discussed the conditional use of CO2 by identifying and converting suitable low-energy resources, such as industrial waste gases, into high-value products like chemicals and fuels.2
Research in the literature has explored the integration of CCU in the chemical industry to reduce Carbon Footprints, fossil fuel depletion, and reliance on fossil feedstocks by incorporating CO2 as an input rather than allowing it to enter the atmosphere.3 Jiang et al. provided an overview of methods for capturing and converting carbon dioxide into synthetic hydrocarbons.4 They highlighted that reusing CO2 emitted from anthropic activities as a raw material for other processes is a significant technological innovation. The approach can potentially stabilize Greenhouse Gas levels in the atmosphere and help mitigate the effects of Global Warming.
In this study, we designed a CCU route based on a hierarchical approach to process synthesis using Aspen Plus simulation software from Aspentech®. We employed the Life Cycle Assessment (LCA) technique to evaluate its environmental performance. In our literature review, we did not find any studies that applied the LCA technique to assess the environmental performance of this route. However, we discovered studies employing environmental metrics to measure Global Warming Potential (GWP) and energy efficiency, which we used to compare our results.
Acetic acid (CH3COOH) has the potential to decarbonize the chemical industry through CCU routes.5,6 It is primarily synthesized from fossil feedstock via methanol carbonylation, a technology that accounts for over 65% of the global production capacity of this acid.7 Alternative methods for producing CH3COOH using CO2 have only been explored in preliminary studies.8 Liquid-phase alkane oxidation plants have gradually decreased production due to their lack of competitiveness compared to CH3OH carbonylation plants, and other technologies still require further development for large-scale production. While these traditional methods are technologically well-established, they lead to significant environmental impacts due to the fossil origins of their raw materials, the energy-intensive nature of the transformations, and greenhouse gas emissions.9
Alternative methods for producing acetic acid include the hydrocarboxylation of methanol10,11 and the hydrogenation of carbon dioxide. Similarly, the CO2 can also be combined with methane or lignin.12 Synthetic (or chemical) processes are the preferred acid manufacturing methods. These routes encompass traditional techniques such as CH3OH or CH4 carbonylation, the acetaldehyde process, liquid-phase oxidation of alkanes, isomerization of methyl formate, and gas-phase reactions of C2H6 and C2H4.13
Because of the environmental impacts of conventional processes, there is a growing interest in developing innovative schemes to synthesize acetic acid. The aim is to reduce the reliance on fossil-based feedstocks and decrease CO2 emissions from anthropogenic processes that utilize non-renewable resources, such as coal and crude oil.14 The flue gases emitted during coal combustion for energy production contain toxic components that can harm human health and contribute to climate change.15 Qian et al.11 investigated this topic by conducting a catalytic methanol hydrocarboxylation experiment using CO2 and H2 to produce acetic acid.11 This reaction was catalyzed by bimetallic Ru-Rh, employing imidazole as the ligand and lithium iodide (LiI) as a promoter in 1,3-dimethyl-2-imidazolidinone. A subsequent study by Cui et al.10 explored the hydrocarboxylation of methanol over a Rh-based catalyst, reducing the amount of LiI by one-third.10 The Aspen Plus software by Aspentech® was used to simulate this process and propose a flowsheet incorporating the operating model conditions. The study confirmed that CH3OH can be converted into acetic acid using CO2 and H2 with high selectivity. According to the mechanism proposed by the authors, the reaction does not proceed via the conventional carbon monoxide (CO) route as in methanol carbonylation. Methyl iodide is produced when LiI reacts with methanol. Under these conditions, oxidative addition occurs at active Rh sites. CO2 is then inserted into the adsorbed species to form CH3COORhI, yielding acetic acid through reductive elimination with H2. The reaction between LiOH and HI also produces LiI and H2O. This promising route employs inexpensive and available feedstocks, such as carbon dioxide, and features a simple catalytic system that is less corrosive and more efficient than previously reported methods. Pacheco et al.16,17 performed a thermodynamic analysis of this reaction to compare traditional and CO2-based innovative acetic acid synthesis routes within the same framework.16,17
Methanol carbonylation depends on fossil resources, specifically CO and CH3OH, and produces various atmospheric emissions during acetic acid production. According to Althaus et al.,18 this process has an environmental performance characterized by a GWP of 1.37 kg CO2eq and a Primary Energy Demand (PED) of 52.6 MJ for each kilogram of acetic acid.18 Medrano-García et al.19 assessed the Carbon Footprint of the CH3OH carboxylation process. They observed values of GWP ranging from 0.10 to 1.83 kg CO2eq per kilogram of CH3COOH, depending on the sources of raw materials, utilities, and, notably, the energy matrix (electricity and heat) of the region where the process occurs.19 As CH3COOH is an intermediate product, the authors argue that reducing its Carbon Footprint and reliance on non-renewable materials can also lower the Global Warming Potential of subsequent products.
In this study, we reported an alternative route for producing acetic acid via methanol hydrocarbonylation using CO2 and H2. The method is promising because it utilizes CO2 as a low-cost and available feedstock, and the rhodium-based catalyst chosen to support the reaction provides competitive efficiency with other processes.10 The route represents an effective way to convert CO2 into high-value-added chemicals, though advancements are still necessary to make it industrially viable. Exploring efficient and straightforward catalytic systems, lowering reaction temperatures, and minimizing corrosive additives are crucial from both scientific and practical perspectives. The study also aims to contribute to this field by quantitatively examining the environmental impacts of coupling CCU technology to various phases of an innovative acetic acid synthesis.
The analysis was conducted in two stages. The first part of the study focused on using the Life Cycle Assessment (LCA) technique to evaluate the environmental performance of methane carbonylation as it occurs in Brazil. The evaluation considered Global Warming Potential (GWP) and Primary Energy Demand (PED) as environmental performance indicators. The results obtained from this assessment were compared to those from similar acetic acid synthesis technologies. This phase also aimed to identify sources of environmental impact, which were then subjected to adaptation measures to reduce, minimize, or eliminate their effects wherever possible.
In the study's second phase, alternative processes developed from the redesign efforts were re-evaluated for their environmental performance using LCA once again. One of these alternative routes involved producing acetic acid through methanol hydrocarbonylation, where CO is replaced by CO2 generated via CCU technology. Resource consumption and emissions for this process were estimated through computer modeling and simulation. The GWP and PED values for these alternative routes were compared with those calculated for the conventional method. We hope this research's findings will contribute to the specification and design of more environmentally friendly methods for synthesizing acetic acid and its byproducts.
The current technology for producing acetic acid via methanol carbonylation is an advancement of the Monsanto Process, which was proposed in the mid-1990s.20 The process involves CH3OH carbonylation facilitated by an iridium catalyst, operating under pressures ranging from 1.0 to 3.0 MPa. Notably, this arrangement achieves an 85% acetic acid yield while demonstrating high catalyst stability, low water consumption, and a reduced formation of liquid by-products compared to the former technology that employs a rhodium catalyst.21
Monsanto process
The Monsanto process, also known as the Celanese Process, begins with water and CH3OH interacting with CO inside a reactor. Under controlled pressure (30 – 40 bar) and temperature (180 °C), this reaction produces acetic acid as the main product and fuel gas as a byproduct. The liquid portion of the reaction mixture is separated from the methanol-water vapors and sent through a series of distillation columns. At the end of this process, the acetic acid is obtained in a form suitable for commercialization.19 Carbon monoxide is introduced into the system in excess, and the unreacted portion is separated from the fuel gas and recirculated back to the reactor via a closed loop. Any losses of carbon monoxide are compensated for by adding syngas. Figure 1 illustrates the flowsheet of the methanol carbonylation system.
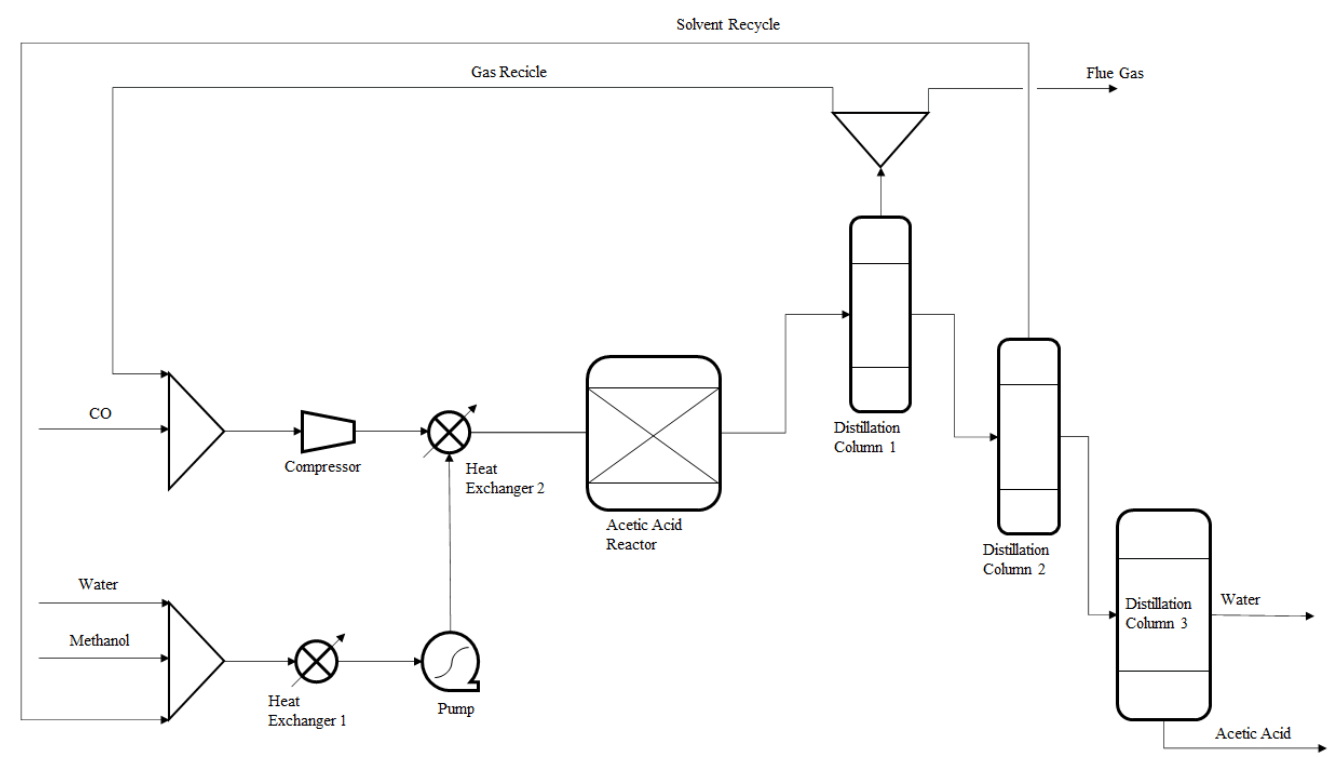
Figure 1 Scheme of Monsanto process (also called the Celanese process) used for producing acetic acid.
Table 1 illustrates the primary material and energy flows involved in acetic acid synthesis through methanol carbonylation. The values presented reflect the reaction's consumption and emission data, as well as the separation steps. In addition to the typical process losses related to CH3OH, CO, and CH4, carbon dioxide is also released into the atmosphere. This emission results from the combustion of natural gas, which is used to meet the arrangement's thermal requirements, particularly within the reaction vessel.
|
Process technology |
Methanol carbonylation |
|
|
Product |
Unit |
Quantity |
|
Acetic acid (CH3COOH) |
kg |
1 |
|
Material Inputs |
Unit |
Quantity |
|
Methanol (CH3OH) |
g |
539 |
|
Carbon monoxide (CO) |
g |
481 |
|
Tap water (H2O) |
g |
154 |
|
Energy Inputs |
Unit |
Quantity |
|
Natural gas |
MJ |
1.39 |
|
Electricity |
Wh |
60 |
|
Atmospheric Emissions |
Unit |
Quantity |
|
Acetic acid (CH3COOH) |
g |
4.99 |
|
Hydrogen (H2) |
g |
0.03 |
|
Carbon dioxide (CO2) |
g |
37 |
|
Carbon monoxide (CO) |
g |
6.31 |
|
Methanol (CH3OH) |
g |
2.52 |
|
Methane, fossil (CH4) |
g |
4.99 |
Life cycle modeling: scope definition and elaboration of the Life Cycle Inventory (LCI)
The environmental performance analysis of the acetic acid synthesis via methanol carbonylation was based on the methodological guidelines outlined in the ISO 14044 standard. In this case, an attributional LCA with a cradle-to-gate scope was adopted to estimate the impact of producing 1.0 kg of CH3COOH (purity ≥ 99% v/v),23 a calculation basis established as the study's Reference Flow. The product system that represents the life cycle of this synthesis route is schematically illustrated in Figure 2. The diagram details the primary flows of raw materials, inputs from their natural resource origins, and the utilities and transportation operations involved.
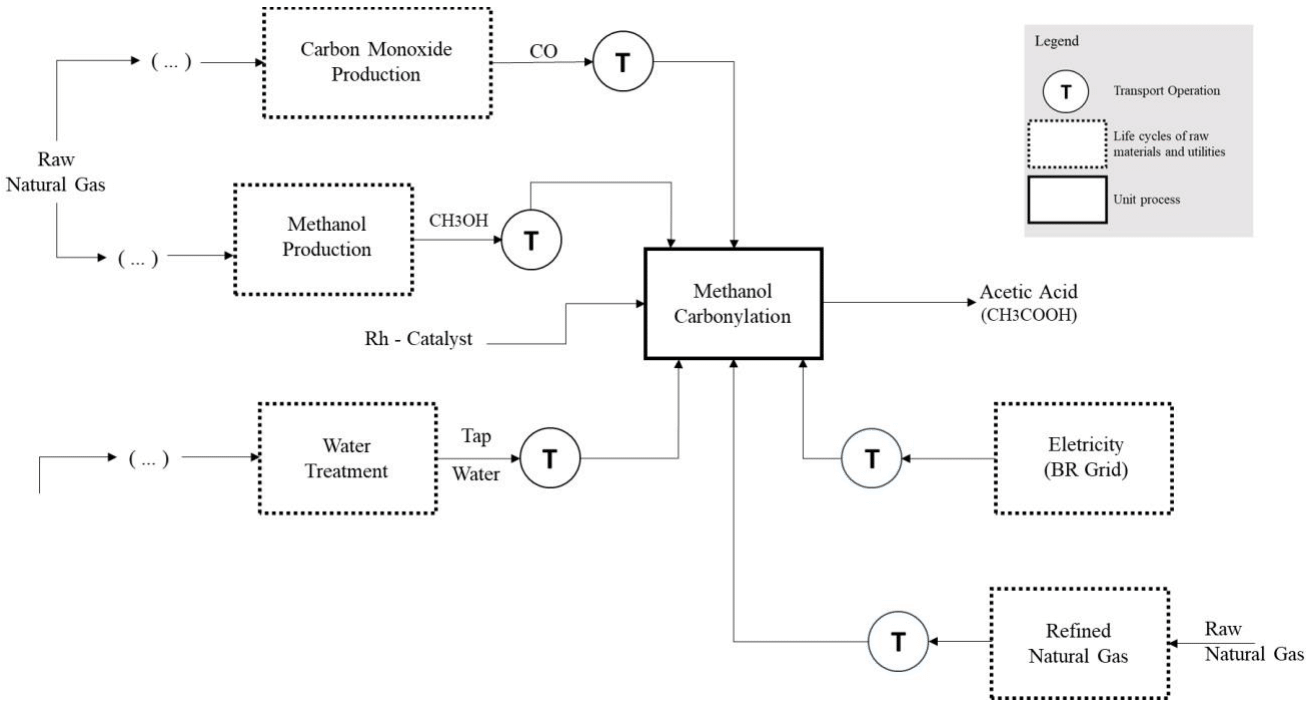
Figure 2 Product system describing acetic acid synthesis from methanol carbonylation technology for LCA purposes.
Concerning the data quality, the temporal coverage for this study covered the entire year (January to December) of 2022. In addition, regarding the geographic coverage, all production units were assumed to be in Brazil. Finally, the technological and operational conditions considered to model methanol carbonylation are summarized in Section 2. The secondary data employed to perform the analysis were collected from official sources.24 These references were used to describe the environmental model for the production route. The process adaptation for Brazilian conditions occurred mainly (but not only) due to the suitability of its utilities (electricity and thermal energy) to that reality. Cut-off criteria were applied to mass and energy flows, excluding any cumulative contributions that were less than 1.0% of the total inputs and outputs from each unit process or subsystem.
The Life Cycle Impact Assessment (LCIA) was developed from two perspectives. The first focused on the environmental impacts of GHG emissions during the entire life cycle, mainly CO2 and CH4. To conduct this analysis, the process's Global Warming Potential (GWP) was estimated using the method proposed by the Intergovernmental Panel on Climate Change (IPCC) in 2013, considering a time frame of 100 years.25 The second perspective examined the process's Primary Energy Demand (PED), calculated using the Cumulative Energy Demand (CED) method – v. 1.11.26 This method differentiates the contributions of non-renewable energy sources (such as fossil fuels, nuclear, and biomass) from renewable sources (including wind, solar, geothermal, biomass, and hydroelectric energy). The PED impact category allowed for quantifying global gross energy consumption associated with methanol carbonylation, characterized by its endothermic nature and high energy intensity.
The Life Cycle Inventory (LCI) was established through specific calculations. After concluding this stage of LCA's methodology, an assessment using SimaPro – v.9.0 software from PRé Sustainability® was carried out to determine the environmental performance of the route. SimaPro estimates environmental impacts through matrix manipulation. To obtain the consolidated LCI, the matrices representing each life cycle stage's environmental loads are multiplied by the technical coefficients' transposed matrices. This adjustment aligns the amounts of the intermediate flows (from each process unit) to the Reference Flow defined for the study. During the Life Cycle Impact Assessment (LCIA) stage, which generates the Environmental Impact Profile (the diagnosis), the matrix that characterizes the consolidated LCI is multiplied by the transposed matrices of the impact factors for the categories selected for the study.27
The consolidated Life Cycle Inventory (LCI) for the methanol carbonylation route was entirely derived from literature data. In this case, mass and energy balances obtained from the Aspen Plus simulation helped harmonize all flows involved. This approach was applied to the production of raw materials (CO and CH3OH), inputs (tap water), and utilities (natural gas, which is used for producing heat and electricity). The original data are sourced from the Ecoinvent database and include the following datasets: 'Acetic acid, without water, in 98% solution state {RoW} | acetic acid production, product in 98% solution state | APOS, U', 'Carbon monoxide {RoW} | production | APOS, U', 'Methanol {GLO} | production | APOS, U', and 'Water, cooling, unspecified natural origin, RoW' (Althaus et al., 2007). The Brazilian electricity network (BR grid) was adjusted according to the average share of energy sources available in 2022.28 At that time, most of the electricity consumed in the country was generated from hydropower plants (62%). That followed this contribution from wind farms (12%), thermoelectric processes using natural gas (6.1%), biomass (i.e., sugarcane bagasse) (4.7%), black liquor (2.5%), coal (1.2%), and the nuclear energy (2.1%).
Environmental performance of acetic acid production from the monsanto process
Table 2 compares the environmental performance of CH3COOH processing through methanol carbonylation, as evaluated using Life Cycle Assessment (LCA) for Brazilian conditions, with findings from the literature. While Althaus et al.18 conducted a similar assessment for European conditions, Medrano-Garcia et al.19 and Roh et al.5 explored the effectiveness of this scheme when it is submitted to innovative practices.18,19,28
|
Impact Category |
Unit (/kg CH3COOH) |
Althaus et al. (2007) |
Medrano-Garcia et al. (2019) |
Roh et al. (2018) |
Methanol carbonylation (Brazilian conditions) |
|
GWP |
kgCO2eq |
1.37 |
0.10 – 1.83 |
2.45 |
1.31 |
|
PED |
MJ |
52.6 |
51.1 |
– |
50.5 |
Table 2 Environmental performance of the conventional acetic acid synthesis route
The authors explored using a superstructure for synthesizing acetic acid, which incorporated various methods for generating syngas, gas separation technologies, and producing CH3OH. Their main intention was to lower overall costs and the GWP impacts of CH3COOH processing.19 Roh et al. also explored the superstructure approach; however, they utilized a computational tool (ArKa-TAC3) designed to facilitate technical, economic, and environmental analyses, explicitly focusing on GWP. Their goal was to identify the most promising configurations in these domains.28
The raw materials significantly impact process performance under typical Brazilian conditions regarding PED and GWP. Methanol is produced by hydrogenating carbon monoxide, which is generated as syngas via the steam reforming of natural gas (CH4 + H2O → CO + 3H2). This reaction is endothermic (Δh = 206 kJ/mol CH4), and the required thermal energy is also supplied by natural gas. The carbon monoxide and methanol processing is energy-intensive, requiring 0.66 kg of heavy fuel oil per kg of CO and 0.82 m³ of natural gas per kg of methanol. These environmental impacts are further attributed to the synthesis of acetic acid. These combined effects account for approximately 94% of the process's global PED. The combustion of fossil fuels contributes to over 73% of the impacts on Global Warming Potential. In this context, the methanol carbonylation stage is particularly noteworthy, as it alone accounts for 21% of the contributions to GWP, making it the primary source of impact in the system.
The comparison between conventional processes indicates that the scheme used under Brazilian conditions is as impactful as that in Europe. There are no significant technological differences, as the historical data on raw material and utility consumption in Brazil (Table 1) shows values that are either similar (carbon monoxide: 480 g/kg acetic acid and methanol: 540 g/kg) or on the same scale (heat: 1.40 MJ/kg; electricity: 57.0 Wh/kg) as the average European figures. This similarity extends to carbon monoxide and methanol synthesis, particularly regarding energy requirements; thermal energy is 6.93 MJ/kg for methanol, and electric energy is 2.30 kWh/kg for methanol and 74.0 Wh/kg for carbon monoxide.
The primary difference between the two regions lies in the fossil nature of the European average energy grid, which is about 91% fossil-fuel-based. This results in a PED) of 8.26 MJ/kWh and a carbon dioxide equivalent (CO2eq) emission of 416 g/kWh. In contrast, the Brazilian grid has a primary energy demand of 5.63 MJ/kWh and emits 247 gCO2eq/kWh.
Medrano-Garcia et al.19 observed that combining Auto-thermal Reforming (ATR) and CO absorption produces 1.83 kg CO2eq per kg of CH3COOH, costing $0.39 per kg. These results could be improved to 1.59 kg CO2eq/kg and $0.28/kg by incorporating a fuel cell and a Reverse Water Gas Shift (RWGS) reactor. This configuration changes the acid synthesis process to Partial Oxidation (POX) and cryogenic distillation. However, it is essential to note that, despite the efforts of Medrano-Garcia et al., the GWP impact of methanol carbonylation in Brazil remains approximately 28% lower than that achieved through the ATR + CO absorption process or even the setup that includes both a fuel cell and RWGS, which has a 17% reduction. The minimum impact – 102 g CO2eq/kg – was reached by combining the fuel cell with RWGS, POX, and Pressure Swing Adsorption (PSA). Nevertheless, in this case, the process cost is nearly three times higher, at $0.98 per kg, compared to using a fuel cell and RWGS scheme.
CO synthesis has always been integrated with acetic acid processing in various situations. This integration impacts the thermal demands of the unit, thereby influencing GHG emissions, primarily from the generation of fossil CO2. The authors also examined the variability of acetic acid production routes. However, these cases still need to address the underlying reaction mechanisms. Even when Medrano-Garcia et al. achieved the best environmental performance among their set of options (fuel cell + WGS + POX + PSA), the improvements appeared to stem from the well-coordinated interaction between the production processes of intermediates and the unit operations used to produce the CH3COOH in each arrangement.19
Roh et al.5 designed a sustainable process for acetic acid production. This arrangement's environmental performance and technical-economic aspects were evaluated using the ArKa-TAC3 tool, specifically adapted for this purpose. A flexible superstructure was created to develop specific models for countries like China, Korea, the UK, and the US. Incorporating CO2 utilization with methanol carbonylation yielded results comparable to other technologies. The Global Warming Potential result attributed to Roh et al.5 (Table 1) is based on the model designed for China. It indicates the performance of the conventional acetic acid synthesis process, which is 2.45 kg CO2eq/kg. Notably, this value is significantly influenced by China's energy grid, where coal is the predominant energy source (accounting for 79%). This factor also helps explain the better results obtained for Brazil.28
The critical aspects identified through the Life Cycle Assessment technique for synthesizing acetic acid via methanol carbonylation in Brazil were utilized to propose alternative processing routes that improve environmental performance. In the second stage of the study, three alternative processes for producing CH3COOH were evaluated. The first option is a variant of the conventional route, where carbon monoxide is obtained from CO2 using Carbon Capture and Utilization techniques. The second route involves using entirely wind energy to meet the process's electric demand, which replaces Brazil's conventional energy matrix. Lastly, the third alternative fully substitutes CO with CO2, which is generated through CCU technology. This approach significantly differs from traditional methods and was developed using computational modeling with various boundary conditions. Sections 3.1 and 3.2 summarize the methanol carbonylation processes using CO2-based CO and the CO2-based manufacturing route generated from CCU.
Methanol carbonylation by CO2-based CO
Conventionally, carbon monoxide is produced through coal gasification, steam reforming, and partial hydrocarbon oxidation processes. However, in the methanol carbonylation process that utilizes CO sourced from CO2, the production of such input shifts away from fossil resources and is instead generated through carbon dioxide hydrogenation.29 To enhance the environmental benefits of this process in terms of Global Warming Potential, it is assumed that the CO2 is obtained through Carbon Capture and Utilization techniques.30
Thonemann and Pizzol31,32 highlight that CO2 is a valuable, environmentally friendly input for CCU technology. While this transformation requires significant energy, using CO2-based chemicals as a clean feedstock can reduce environmental impacts compared to traditional methods. Furthermore, CO produced through CCU technology demonstrates competitive environmental performance relative to conventional production methods, regardless of the energy grid used.31,32
Regarding technology, CO2-based carbon monoxide production occurs through the Reverse Water Gas Shift (RWGS) reaction, where H2 and CO2 react, as shown in equation (Eq. 1).31,33 Additionally, a reaction occurring during RWGS involves CO2 methanation (equation Eq. 2), which results in lower CO yields.34
𝐶𝑂2 + 𝐻2 ⇋ 𝐶𝑂 + 𝐻2𝑂 (1)
𝐶𝑂2 + 4𝐻2 ⇋ 𝐶𝐻4 + 2𝐻2𝑂 (2)
An active RWGS catalyst must be used and operated at low temperatures while maintaining high CO selectivity to prevent reduced CO yields. Zhu et al.34 reviewed metal catalysts that enhance the conversion and selectivity of the RWGS process, concluding that gold (Au) and platinum (Pt) catalysts can improve the selectivity of the RWGS reaction. Conversely, innovative catalysts based on ruthenium (Ru) and rhodium (Rh) are more favorable for CH4 formation, which results in decreased CO yields in this reaction.34
CO2-based Manufacturing route
The CO2-based manufacturing route – also called the Methanol hydrocarboxylation route or the CCU process – utilizes CO2 as a raw material to replace fossil CO in producing acetic acid. This method is under development and has not yet reached a high level of technological maturity. Anyway, two studies have provided experimental data for the methanol hydrocarboxylation process. Qian et al.11 proposed a reaction involving methanol, CO2, and H2, catalyzed by a bimetallic Ru-Rh homogeneous catalyst. In this study, imidazole served as the ligand, lithium iodide (LiI) was used as a promoter, and 1,3-dimethyl-2-imidazolidinone was the solvent. In another study, Cui et al.10 employed an Rh-based catalyst that reduced LiI consumption by one-third while achieving a higher acetic acid yield. Due to these promising results, the latter experimental data will be utilized in this research as the reaction system.10,14
To simulate the process using a commercial simulator, such as Aspen Plus, there are several approaches for the reactor system:
Given the lack of kinetic data for this reaction system in the literature, we have chosen the stoichiometric reactor for our evaluation. It is important to note that while this approach produces estimated results for the reactor, it also aligns with the level of detail needed for a prospective analysis, which is the focus of this study for a conceptual plant.
Using Aspen Plus v9.0 software from Aspentech®, we simulated the plant to establish a robust and technologically consistent production scheme for this method (Figure 3). The algorithm proposed by Smith and Missen35 was employed to simultaneously determine the number of independent equations and a complete set of chemical equations.35 The chemical system consists of CH3OH, CO2, H2, H2O, CH3COOH, CH4, C2H5OH, C3H6O2, and C4H8O2. An additional constraint was applied: CO2 and H2 react in equal amounts under certain conditions.10 This constraint has been explicitly included in the general description. A complete stoichiometric matrix in the canonical form is presented in Equations Eq.3 to Eq.7
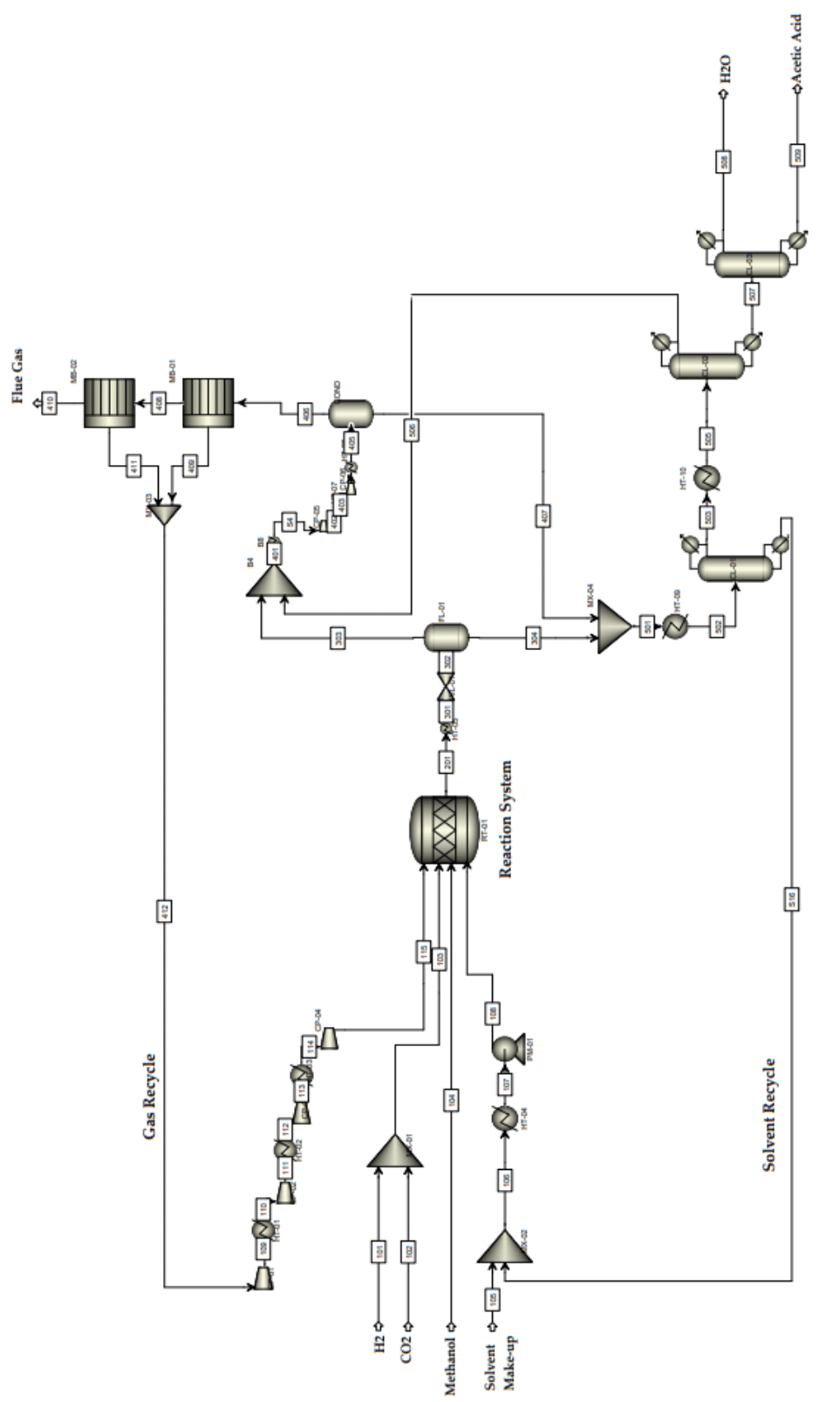
Figure 3 Proposed flowchart for acetic acid production via the methanol hydrocarboxylation route (the CCU process).
𝐶𝐻3𝑂𝐻 + 𝐶𝑂2 + 𝐻2 ⇋ 𝐶𝐻3𝐶𝑂𝑂𝐻 + 𝐻2𝑂 (3)
3𝐶𝐻3𝑂𝐻 ⇋ 2𝐶𝐻4 + 𝐶𝑂2 + 𝐻2 + 𝐻2𝑂 (4)
2𝐶𝐻3𝑂𝐻 ⇋ 𝐶2𝐻5𝑂𝐻 + 𝐻2𝑂 (5)
2𝐶𝐻3𝑂𝐻 + 𝐶𝑂2 + 𝐻2 ⇋ 𝐶3𝐻6𝑂2 + 2𝐻2𝑂 (6)
3𝐶𝐻3𝑂𝐻 + 𝐶𝑂2 + 𝐻2 ⇋ 𝐶4𝐻8𝑂2 + 3𝐻2𝑂 (7)
The conversion for Eq. 3 was adjusted using the logistic curve from the experimental data provided by Cui et al.10 as a function of temperature.10,11 The primary side reaction involved the production of CH4 (Eq. 4) and was also adjusted based on experimental results. An Rh-based catalyst, the 1,3-dimethyl-2-imidazolidinone, is added to the process to enhance the reaction speed and increase selectivity for acetic acid.10 This catalyst acts as a solvent in the simulation. It is continuously recirculated in the reactor and periodically replenished to replace losses or mitigate passivation effects. The reaction occurs at 180 ºC and 100 bar, producing an overall process yield η ~ 82% for CH3COOH.
After several purification stages, the acetic acid attains a purity level of about 99.5%, surpassing even that of its counterparts synthesized via conventional production methods. A gas separation system utilizing polymeric membranes recovers most of the unreacted CO2 (94% v/v) and H2 (99% v/v), reintegrating these compounds as raw materials in the plant. The gaseous flow exiting the second separation column, which contains 92% v/v CO2, is also returned to the reaction vessel. Additionally, the process yields a byproduct, fuel gas primarily composed of CH4, which can be utilized as an energy source in processes adjacent to the CH3COOH unit.
The unit is designed to achieve a production capacity of 200,000 tons per year. For most chemical and petrochemical processes, plant operation typically accounts for between 90% and 95% of the total hours in a year (8,760 hours). Furthermore, CO2 is a readily available feedstock, and the CCU route employs a straightforward catalytic system that is less corrosive and more efficient than methanol carbonylation and its variants.
The process encompasses five main areas:
Pettersen and Lien36 proposed a model to simulate the membrane module for the gas separation system. This model uses an analogy with the fundamental equation of heat exchangers as a shortcut design method for a hollow fiber module in counter-current operation.36 Several authors, including Gassner et al.,37 Morosanu et al.,38 and Nguyen and Oliveira39 have utilized the same model to simulate the membrane module.37–39 The permeability and selectivity values for the polyimide membrane were obtained from the work of Abetz et al.40 The outlet stream from the membrane module is recompressed before being fed into the reactor.
The physical properties of acetic acid are well documented concerning thermodynamic models. Due to molecular associations, some carboxylic acids, including the CH3COOH, can dimerize in the vapor phase. The dimerization leads to a high degree of non-ideality for the gas phase. The Hayden-O'Connell equation accounts for this effect in fugacity calculations for the gas mixture, which provides the best predictions for vapor-liquid equilibrium (VLE) experimental data.41 Consequently, the NRTL-HOC thermodynamic model was selected for the simulation.
Under the specified design conditions, 16.3 tons per hour (t/h) of CH3OH reacts with 27.3 t/h CO2 and 800 kg/h H2 in a solvent medium to yield 25.0 t/h CH3COOH. The gas separation system extracts condensate using compressors (12.6 MW), chillers (7.40 MW), and a membrane module (at a pressure drop of ΔP = 23.9 bar). The cumulative result of these processes is a stream consisting solely of hydrogen and carbon dioxide in a ratio of [58:42]p/p, which requires 14.8 MW to be recompressed. These performance metrics are comparable to those obtained by Feyzi and Beheshti42 under similar operating conditions.42
Aspen Plus v9.0 facilitated accurately characterizing the most likely operating conditions for the CCU system. The software ensured the convergence of the mass and energy flows involved in the scheme by calculating them based on physical, chemical, and thermodynamic data packages. Ultimately, this computational approach favored the harmonization of consumptions and emissions within the system, considering the data used for modeling the synthesis of raw materials and inputs and those describing the generation of associated utilities.
Scenario definition and formulation of assumptions for the LCA application
The production routes for acetic acid under consideration were organized into different study scenarios to allow for an adequate assessment of environmental performances. The conventional process is designated as the baseline scenario (S1). Table 3 outlines this organization and specifies the details of each arrangement.
|
Scenario/Requirement |
S1 |
S2 |
S3 |
S4 |
|
Technological approach |
Methanol Carboxylation |
Methanol Carboxylation |
Methanol Carboxylation |
Methanol hydrocarboxylation (CDU route) |
|
Use of CO as raw material |
(+) |
(+) |
(+) |
(–) |
|
Origin of CO |
Fossil resources |
CO2 |
CO2 |
N/A |
|
Use of CCU technology |
(–) |
(+) |
(+) |
(+) |
|
Electricity source |
BR grid |
BR grid |
100% wind |
BR grid |
Table 3 The main specificities of the study scenarios describe the conventional and alternative routes for acetic acid production
Legend: (+): condition met; (–): condition not met; N/A: not applicable
The LCA technique was applied in this study stage according to the guidelines established for determining the impact profile of S1. This involved conducting an attributional cradle-to-gate LCA to produce 1.0 kg of high-purity acetic acid. The data used for the analysis were sourced from secondary origins, ensuring they met the quality requirements established in the previous stage, particularly in terms of temporal and geographic coverage. Each route followed the specifications outlined in sections 3.1 and 3.2 regarding technology. No multifunctional situations were identified in processes S2 and S3, and the multifunctional scenarios observed in S4 were addressed by applying the allocation procedure, with energy content as the criterion.
The stream that used CO2 as a raw material was treated as elementary flows, meaning the evaluation did not consider any associated environmental impacts from their generation. This approach was informed by Curran43 and von der Assen et al.,44 which suggest that CCU practices are strategies for valuing this greenhouse gas.43,44 The LCA methodology's Life Cycle Impact Assessment (LCIA) step was again carried out to evaluate Global Warming Potential and Primary Energy Demand. Figure 4 illustrates the production systems representing scenarios S2 through S4.
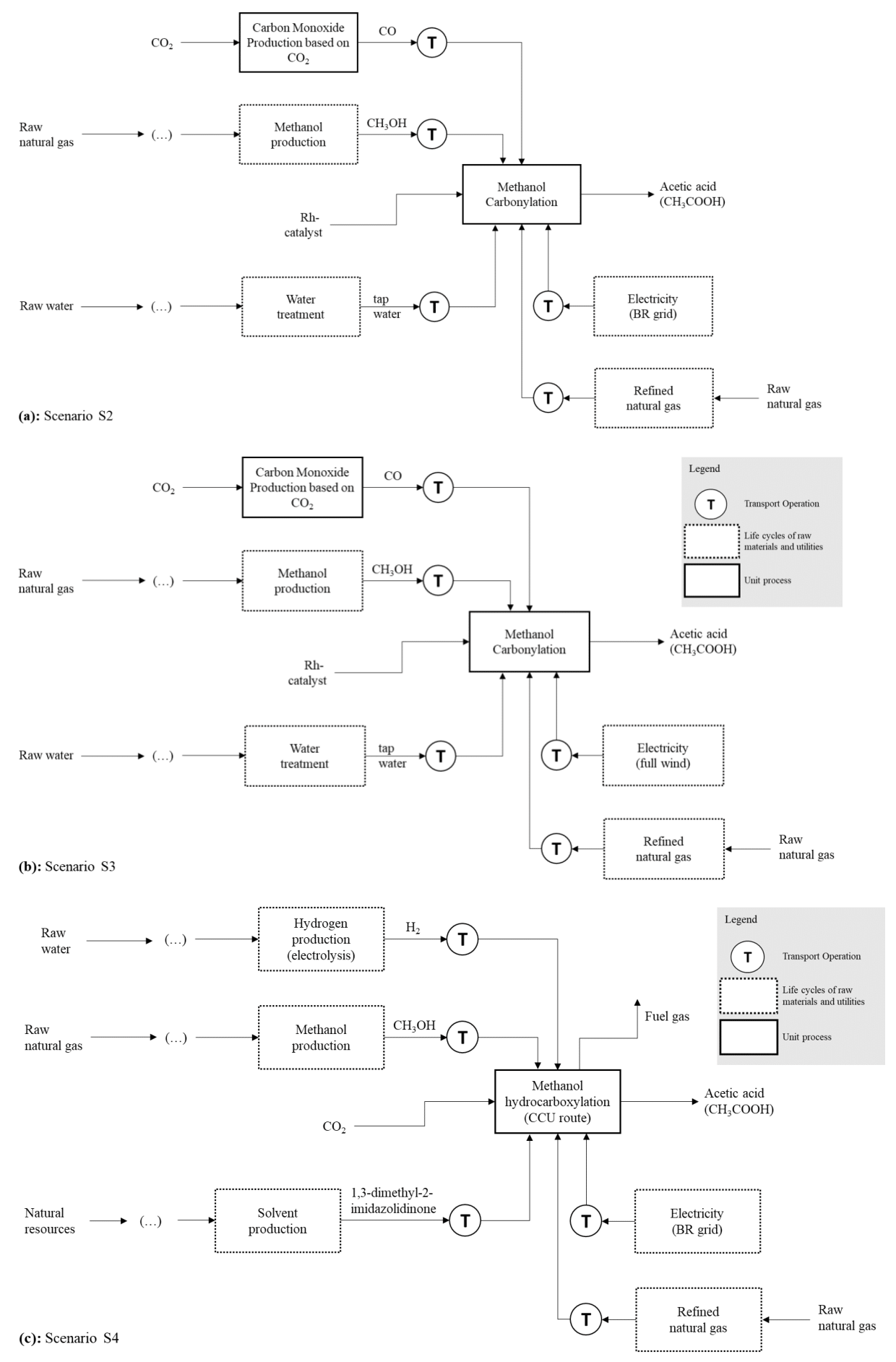
Figure 4 Product systems for synthesizing acetic acid through (a) Methanol carbonylation by CO2-based CO (S2), (b) Methanol carbonylation by CO2-based CO and wind electricity (S3), and (c) methanol hydrocarboxylation (the CCU process) (S4).
The conversion of CO2 into CO was designed to integrate the model that describes S2. The carbon dioxide in this transformation comes from ammonia processing, which provides CO2 that can be used directly in downstream processes without further purification. Adapted versions of the following Life Cycle Inventories from the Ecoinvent Database were utilized to construct the same scenario: ‘Hydrogen gas, from membrane technology, at plant/RER Mass’, ‘Carbon dioxide, liquid {RER}| market for | APOS, U', 'Methanol {GLO}| production | APOS, U', ‘Chemical factory, organics {GLO}| market for | APOS, U’, 'Carbon monoxide {RoW}| production | APOS, U', ‘Nitrogen, liquid {RoW}| air separation, cryogenic | APOS, U’ and ‘Water, decarbonized, at user {RoW}| water production and supply, decarbonized | APOS, U’.18 The electricity mix for S3 consists entirely of wind energy.45s
Finally, regarding S4, CO2, H2, and CH3OH production, the synthesis of 1,3-dimethyl-2-imidazolidinone and the generation of utilities were also included. The manufacturing of H2 was intended to be carried out through electrolysis. To support this process, the datasets for ‘Hydrogen gas, from membrane technology, at plant/RER Mass’, ‘Methanol {GLO}| production | APOS, U’ and ‘Carbon dioxide, liquid {RER}| market for | APOS, U' also from Ecoinvent were adapted to the scenario.46 The CO2 utilized in the CCU synthesis of acetic acid is derived from ammonia processing. The natural gas production cycle was modeled using the same approach, which includes extraction (both onshore and offshore) and refining and distributing the finished product. This information can be found in the “Natural gas, at production / RER’ dataset.47
Table 4 shows the performance of scenarios S1 to S4 for acetic acid production, focusing on Global Warming Potential and Primary Energy Demand. The results reveal that integrating CCU practices with the methanol carbonylation process can offer environmental advantages over the conventional setup. In this context, CCU emerges as a promising approach. Even without modifications to enhance its technical performance, coupling it with the traditional method led to a decrease in contributions to GWP compared to the conventional arrangement.
|
Impact category |
Unit (/kg CH3COOH) |
S1 (baseline) |
S2 |
S3 |
S4 |
|
GWP |
kg CO2eq |
1.31 |
1.29 |
1.2 |
0.69 |
|
PED |
MJ |
50.5 |
30.9 |
29.3 |
18 |
Table 4 Environmental performance of the conventional acetic acid and CO2-based improvement proposals for acetic acid synthesis
For the conventional route utilizing partial CCU (S2), CO2-based CO was used. The carbon monoxide is produced through the reaction: CO2 + H2 → CO + H2O. The reaction is endothermic (ΔH = 41.5 kJ/mol) and, like any CCU-CO2 conversion reaction, is highly energy-intensive, requiring 8.0 MJ for each kilogram of CO produced. CH3OH is still made via CO hydrogenation, similar to the baseline scenario. However, only a modest improvement of 1.5% in GWP was observed compared to S1. In contrast, a significant reduction of 39% was noted in the PED category. This gain is attributed to decreased fossil fuel contributions, which dropped from 49% in S1 to 17% in S2. If CO2-based methanol is also considered, the results could compete with S4.
When the BR grid is replaced exclusively with wind power, there is a 7.0% reduction in GWP impacts for S3 compared to S2. In the same comparison, the improvement in PED is even less significant at approximately 5.2%. These results suggest that electricity consumption is not a considerable factor affecting the environmental impact of processing acetic acid in Brazil, primarily due to the nature of the national grid, which consisted of around 88% renewable energy sources in 2022.48 In S4, the hydrogen consumed for synthesizing CH3COOH and CH3OH is generated through electrolysis technology. Although this process has a high electricity requirement (18.4 kWh/kg),49 it results in lower PED impacts than the conventional route due to the significant proportion of renewable resources in the Brazil 2022 grid.
The CCU process utilizes exhaust gases from the ammonia industry, which contain, on average, 96% w/w of CO2, eliminating the need for purification. While capturing and using this raw material requires energy, it is generally less harmful to the environment than producing the raw materials needed for the conventional route. Additionally, another source of environmental benefit arises from substituting CO for CO2.
Regarding the conditions in Brazil, this adjustment led to performance improvements of 64% for Primary Energy Demand and 47% for Global Warming Potential compared to the indices observed in S1. These results further emphasize that the accumulated demand for primary energy and the greenhouse gas emissions associated with the carbon monoxide life cycle are higher than those of the alternative provided by CCU coupling. Under these circumstances, CO2 changes its role, shifting from an atmospheric emission of the NH3 synthesis process to an input for acetic acid production. As a result, the environmental impacts generated alongside its output are not included in this assessment.
Considering the environmental results from the previous section regarding the methanol hydrocarboxylation route, we observed that the reactor plays a vital role in the process. Studying extreme conditions is essential in this picture, as the data obtained are critical for environmental assessments. Therefore, a sensitivity analysis was conducted. To perform it, the reactor conditions were modified, and the impact on the global environmental performance of the system was estimated. Literature data on acetic acid yield at different temperatures for the methanol hydrocarboxylation reaction were fitted to a logistic regression curve.10 The final expression was incorporated into the Aspen Plus simulation as a Fortran code.50 The acetic acid yield in the reactor was then determined based on the reactor's temperature.50 Results from this analysis are presented in Figure 5.
The temperature range of 190 to 200 ºC shows only a slight increase in yield (1.28% and 1.35%, respectively) compared to 180 ºC. However, the energy required to raise the temperature by 10 – 20 ºC is neither environmentally nor economically feasible. In this region, the yield curve behaves asymptotically, suggesting that 180 ºC can be utilized as an extreme value. Conversely, lower temperatures, such as 170 ºC, decrease the required thermal energy, resulting in lower contributions to Global Warming Potential and Primary Energy Demand. However, this also leads to a 21% decrease in acetic acid yield, which could impact the separation train (distillation columns) and the recycled streams (pumps and heat exchangers).
After conducting this analysis, we concluded that reducing temperatures below 175ºC does not improve environmental metrics due to the reactor's reduced use of thermal energy. However, more power is needed in other parts of the process, and acetic acid yields decrease.
This study assessed the environmental performance of Carbon Capture and Utilization (CCU) practices for producing acetic acid, intending to enhance its environmental sustainability. The method, which involves the hydrocarboxylation of methanol using CO2 sourced from ammonia synthesis, was simulated using Aspen Plus software, considering Brazilian conditions. This approach was compared to the conventional carbonylation route for methanol production. An Attributional Life Cycle Assessment (LCA) was conducted, covering a cradle-to-gate analysis for producing 1.0 kg of acetic acid with a purity of 99% v/v+. The results were expressed regarding Global Warming Potential (GWP) and Primary Energy Demand (PED).
The findings indicate that integrating CCU practices into the acetic acid synthesis process can lead to significant environmental benefits, reducing GWP and PED impacts compared to traditional methods. Energy consumption, including electricity and heat, was identified as a critical factor, with the conventional route (S1) resulting in GWP impacts of 1.31 kg CO2 equivalent per unit of raw feed (RF) and a PED of 50.7 MJ per RF.
Substituting CO with recovered CO2 resulted in a reduction of impacts: 1.5% for GWP and 39% for PED in scenario S2, 8.4% for GWP and 42% for PED in S3, and 47% for GWP and 64% for PED in S4 compared to the conventional process. This joyous performance is further validated by comparisons with other studies that have evaluated the environmental impacts of CCU practices in producing the same organic acid.
Moreover, utilizing natural gas combustion for heat supply accounted for 40% of the environmental load for GWP linked to the combination of CCU and acetic acid synthesis. Implementing energy integration strategies in this system could mitigate these effects, resulting in improvements in PED. One viable approach could involve integrating fuel gas into the acetic acid production unit to minimize the need for external heat, thereby lowering production costs and enhancing the competitiveness of acetic acid production.
Future directions
This study was part of a larger project to assess promising chemical pathways using carbon dioxide as a raw material. The initial step of this framework involved producing a chemical that aligns with the proposed flowsheet while ensuring compliance with environmental standards. For this analysis, we evaluated the different routes based on environmental metrics.
The newly proposed flowsheet presents specific challenges for large-scale implementation compared to a well-established route. Once the new route's environmental advantages are demonstrated, further research is necessary to enhance its efficiency through optimization, intensification, and heat integration.
After the initial assessment and comparison of processing routes at the proposed level of detail, the next step will be to include additional parameters in the simulation to increase its robustness. It is also essential to highlight the critical significance of this study in selecting the most promising routes for further evaluation.
The authors gratefully acknowledge the support of the RCGI – Research Centre for Gas Innovation, hosted by the University of São Paulo (USP) and sponsored by FAPESP – The São Paulo Research Foundation (2014/50279-4) and Shell Brasil. The authors also acknowledge FAPESP for the Ph.D. scholarship grant (FAPESP Process 2017/26683-8). This study was also financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/Coordination for the Improvement of Higher Education Personnel - Brazil (CAPES) - Finance Code 001.
None.
The authors declare no conflict of interest in writing the manuscript.

©2024 Valdés, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.