MOJ
eISSN: 2573-2919


Research Article Volume 1 Issue 1
Department of Advanced Zoology & Biotechnology, Govt Arts College (Autonomous), India
Correspondence: Elumalai Kuppusamy, Department of Advanced Zoology & Biotechnology, Govt Arts College (Autonomous), Nandanam, Chennai -600 035, Tamilnadu, India
Received: September 01, 2016 | Published: September 29, 2016
Citation: Elumalai K, Kasinathan ID. Antioxidant activity and phytochemical screening of different solvent extracts Cluasena excavate burm F. (Rutaceae). MOJ Eoc Environ Sci. 2016;1(1):1-6. DOI: 10.15406/mojes.2016.01.00001
The aim of this study was to screen different solvent extracts of Cluasena excavata (leaves) to display potent antioxidant activity and also the total phenolic and flavonoid contents in order to find possible sources for future novel antioxidants in food and pharmaceutical formulations. Measurement of free radical scavenging activity (RSA) [reactions with 1,1-diphenyl- 2-picrylhydrazyl radical (DPPH), Nitric oxide scavenging assay by using UV-double beam spectrometer. Among the different extracts tested, the methanol extract showed significant antioxidant activity. Medicinal plants are widely spread in Tamil Nadu and their biological and phytochemical properties are not thoroughly evaluated. In this present study, crude extract of Cluasenaexcavation (leaves) were screened to regulate their active chemical constituents using conventional chemical tests (precipitation and color reagents) and antioxidant activities were determined using the DPPH radical scavenging method. The phytochemical tested shows the presence of various secondary metabolites such as alkaloids, flavonoids, glycosides, phenols, Terpenoids, saponins, and tannins etc. The plants revealed promising antioxidant activity, and require further studies to throw light on their chemical composition and antioxidant properties.
Keywords: clausena excavate, DPPH; FRAP, NO2, UV absorption, ROS
Now-a-days, there is an increased occurrence of various diseases like cardiovascular disease, neurological disorders, cancer, diabetes and autoimmune disease due to the presence of free radicals. Bandyopadhyay et al.1 Antioxidant is defined as the agent that neutralizes the effect produced by free radical Fang Y et al.2 The antioxidant can be classified into two categories, namely enzymatic and non-enzymatic. The enzymatic antioxidants are produced itself in our body, whereas most of non-enzymatic antioxidants are obtained from either natural plants or synthetics which are used for the treatment for various diseases Lee J et al.3 and Cuzzocrea S et al.4 A number of side effects like liver damage and mutagenesis are associated with the use of antioxidants obtained from synthetic sources Grice HC.5 Thus, researchers are exploring natural resources to find out newer and safer natural antioxidants. The potentially reactive derivatives of oxygen, attributed as reactive species (ROS), are continuously generated inside the human body. The generated ROS are detoxified by the antioxidants present in the body. However, overproduction of ROS and/or inadequate antioxidant defense can easily affect and persuade oxidative damage to various bimolecular, including proteins, lipids, Natural antioxidants lipoproteins and DNA. The WHO estimates that up to 80% of people still rely mainly on traditional remedies such as herbs for their medicine.6 India has been identified as a major resourceful area in the traditional and alternative medicines globally. Multi-factorial health beneficial activity of these plant extracts has been attributed to multi-potent anti-oxidant, anti-microbial, anti-cancer, anti-ulcerative and anti-diabetic properties. Several medicinal plants (Rasayana) have also been extensively used in the Indian traditional (Ayurveda) system of medicine for the treatment of number of diseases. Some of these plants have shown potent antioxidant activity. Kaur C et al.7 and Aqil et al.8 The WHO estimates that up to 80% of people still rely mainly on traditional remedies such as herbs for their medicine Tripathi et al.6
Currently, the search for plant sources of antioxidants is gaining momentum with Clausena excavata (Rutaceae family) among the plants targeted. C. excavata is a medicinal plant widely distributed in Southeast Asia and is known by unique local names, such as Chamat in Thailand and Jia huang pi in China. In Malaysia the plant is locally known as “Cherek hitam” and “Kemantu hitam. In Tamil Kattukaruveppilai or Kattu veppilai. ”The leaves of the plant are used in folklore medicine for the treatment of several illnesses such as malaria, headache, abdominal pain, dysentery, pulmonary tuberculosis, diarrhea, cold, wound, snakebite, and poisoning. Recent studies showed that the plant also possessed immune-modulator Manosroi et al.,9 analgesic Rahman et al.10 anti- inflammatory, antivirus, anticancer,11 antioxidant Guntupalli et al.12 antimycobacterial Sunthitikawinsakul et al.,13 and antifungal14 activities. C. excavata has been reported to exhibit one of the highest beneficial biological activities among Clausenagenus Arbab et al.15
Source of plant materials
Leaves of Clausena excavata, were collected from Yercaud hills, Salem District, Tamilnadu, India. The collected plant specimen was identified by Taxonomist from the Department of Botany, Govt. Arts College (Autonomous), Chennai-600035, Tamil Nadu, India and The Voucher specimen was deposited in the laboratory.
Preparation of plant extracts
The dried leaves (100g) were powdered mechanically using commercial electrical stainless steel blender and extracted sequentially with hexane, ethyl acetate and methanol (500ml, Ranchem), in a soxhlet apparatus separately until exhaustion. The extracts were individually concentrated under reduced pressure of 22-26mm hg at 45° C by ‘Rotavapour’ (SUPERFIT PBU-6D) and the residue obtained was stored at 4°C in an amber vial. Then the vials were named and covered with silver foil and transported to the laboratory. Until use those vials were kept in cool and dark place at 4°C.
Antioxidant activity - DPPH (2, 2-diphenyl-1-picrylhydrazyl) radical scavenging assay
The Radical Scavenging Activity (RSA) of different extracts was determined by using DPPH assay following the method of Chang et al. [16] with small modification. The decrease in the absorption of the DPPH solution at 517nm after the addition of antioxidant was measured in a cuvette containing 2.960μl of 0.1 mm methanolic DPPH solutions; 40μl of 20 to 200μg/mL of plant extracts after vortexing the mixture thoroughly. The setup was left at dark in room temperature and the absorption was monitored after 20 minutes by using double beam UV spectrophotometer. Ascorbic acid and Butylated hydroxytoluene (BHT) were used as references. The ability of the plant extract to scavenge DPPH radical was calculated by the following equation
![]()
Where Ac=Absorbance of control; As=Absorbance of sample
Abs. control = Absorbance of DPPH radical + ethanol; Abs. sample = absorbance of DPPH radical + plant extract. Measurements were performed in triplicates. Absorbance values were corrected for radicals decay using blank solutions.
Ferric reducing antioxidant power (FRAP) assay
FRAP reagent (1.8 ml) was mixed with 0.2ml of test sample, then incubated at 37°C for 10 minutes in a water bath. The FRAP reagent contains 20mM TPTZ (2, 4, 6-tri(2-pyridial)-S-triazine) solution 20mM FeCl3, 6H2O and 0.3m Acetate buffer with pH 3.6. After incubation the absorption were measured immediately at 593nm. The above mixture without the plant extract served as control. Methanolic solution of known Fe (II) concentration was used as standards. The values were expressed as mmol Fe (II)/g dry weight of the extract. The assay was carried out in triplicate and the mean values with ± S. D are presented.
Nitric oxide scavenging assay
The nitric oxide radical inhibition was estimated by the use of Griess Illosvoy reaction Garrat17 for dichloromethane, ethyl acetate and methanol extract of Clausena excavata with positive control and standard. The negative control was also prepared with distilled water and the reagent. The absorption was measured 540nm then the % of inhibition was calculated.
![]()
Where Ac=Absorbance of control; As=Absorbance of sample
Estimation of flavonoids
The flavonoids content was determined as follows: 0.1ml of sample method Markhamet al.18 (1mg/ml) was mixed with 2g ml of 10% aluminum chloride and 0.1ml of sodium acetate (1M). To this mixture, 4.3ml of 80% methanol was added to make 5ml volume. This mixture was vortexed and the absorbance was measured spectrophotometrically at 415nm. The value of optical density was used to calculate the total flavonoid content present in the sample.
Estimation of total phenolic content
Total phenolic content was estimated using the 0.5ml Folin-reagent method Harborne et al.19 Samples (100μl) were mixed thoroughly with 2 ml of 2% Na2CO3. After 1 min. 100 μl of Folin-Ciocalteu reagent was added to the mixture. The resulting mixture was allowed to stand at room temperature for 30 min and the absorbance was measured at 650 nm against a blank. Total phenolic content was expressed as gram of gallic equivalents per 100 gram of dry weight of the plant samples.
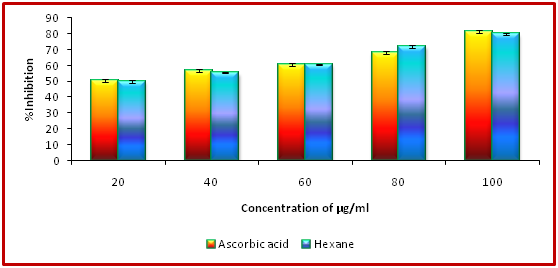
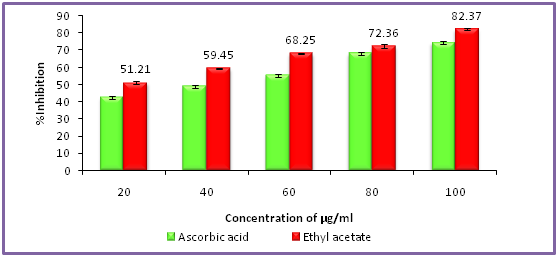
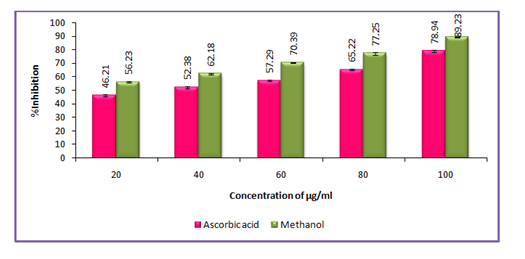
Antioxidant activity (DPPH assay) assay of hexane, ethyl acetate and methanol extracts of Clausena excavata
Antioxidant activity with reference to 2,2-diphenyl-1-picrylhydrazyl assay of hexane extract of Clausena excavata was tested with concentration ranging from 10 - 100 µg/ml as shown in Figure 1. The data pertaining to the activity clearly revealed that the percentage of antioxidant activity was ranged from 49.90% to 79.90%. Similarly, the antioxidant activity with reference to 2, 2-diphenyl-1-picrylhydrazyl assay of ethyl acetate extract of C. excavata was tested with concentration ranging from 10 - 100 µg/ml as shown in Figure 2. The data pertaining to the activity clearly revealed that the percentage of antioxidant activity was ranged from 51.21% to 82.37%.In the same way, the antioxidant activity of 2,2-diphenyl-1-picrylhydrazyl assay of methanol extract of C. excavata was tested with concentration ranging from 10 - 100 µg/ml as shown in F. The data pertaining to the activity clearly revealed that the percentage of antioxidant activity was ranged from 56.23% to 89.23%.
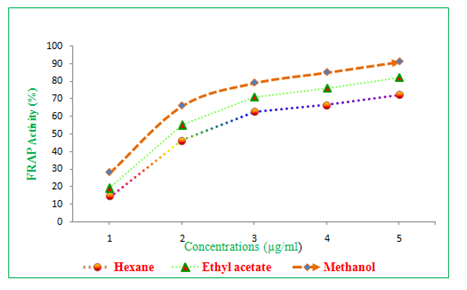
Antioxidant activity (FRAP) of hexane, ethyl acetate and methanol extracts of Clausena excavata
Antioxidant activity (FRAP) of different solvent extracts of C. excavatawas tested and the data observed were depicted in the Table 1. It was observed that the FRAP activity was ranged from 12.42 to 68.22% hexane extract of C. excavata. In the same way, the ethyl acetate extract of C. excavataleaf showed 16.22 to 72.12. Whereas, the maximum antioxidant activity (FRAP) was recorded in the methanol extract and the percentage was ranged from 18.42 to 82.22% (Table 1 & Figure 4).
Concentrations Tested |
Solvents Tested |
||
|---|---|---|---|
Hexane |
Ethyl Acetate |
Methanol |
|
% of Inhibition |
|||
20 |
12.42±1.16 |
16.22±2.00 |
18.42±1.62 |
40 |
38.12±2.18 |
44.38±2.11 |
48.36±1.00 |
60 |
44.24±4.31 |
54.12±3.18 |
62.32±1.74 |
80 |
52.38±3.00 |
62.22±2.02 |
72.18±2.84 |
100 |
68.22±3.11 |
72.12±2.08 |
82.22±3.16 |
Table 1 Antioxidant activity (FRAP assay) of different solvent extract of Clausena excavata leaf.
Values represent mean ± S.D of three replications. FRAP-Ferric Reducing Antioxidant Power.
Antioxidant activity (NO2) of hexane, ethyl acetate and methanol extracts of Clausena excavata
Antioxidant activity (NO2) of different solvent extracts of Clausena excavata was tested and the data observed were depicted in the Figure 2. It was observed that the NO2 activity was found to be 14.25 and 72.39. % in the hexane extract of C. excavata. Similarly, the ethyl acetate extract of C. excavataleaf showed 19.33 and 82.17%. Whereas, the maximum antioxidant activity (NO2) was recorded in the methanol extract and values were found to be 28.37 and 91.25% in 20, 40, 60, 80 and 100μg/ml concentrations respectively (Table 2 & Figure 5).
Concentrations Tested |
Solvents Used |
||
|---|---|---|---|
Hexane |
Ethyl Acetate |
Methanol |
|
% of Inhibition |
|||
20 |
14.25±2.34 |
19.33±1.17 |
28.37±1.08 |
40 |
46.29±1.21 |
55.29±3.67 |
66.23±3.87 |
60 |
62.38±2.87 |
71.26±2.47 |
79.24±1.28 |
80 |
66.47±2.39 |
76.21±3.54 |
85.21±3.25 |
100 |
72.39±2.33 |
82.17±1.28 |
91.25±2.14 |
Table 2 Antioxidant activity (NO2scavenging assay), of different solvent extract of Clausena excavata leaf.
Values represent mean ± S.D of five replications. NO2: Nitric oxide.
Phytochemical screening of hexane, ethyl acetate and methanol extracts of C. excavata
The phytochemical screening of selected plant extracts was assessed and the results pertaining to the experiments are shown in Table 3. The observations with regards to this, clearly indicated that the presence of coumarins alone in the ethyl acetate and methanol extract of C. excavata. Whereas, the presence of alkaloids, flavonoids, glycosides, phenolics, annins, saponins and coumarins in the ethyl acetate extract of the same plant. Similarly, the methanol extract showed the presence of alkaoilds, flavonoids, saponins, tannins, coumarins, Glycosides and phenolics (Table 1).
Phytochemical Screening |
Extracts Tested |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Hexane |
Ethyl Acetate |
Methanol |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Alakaloids |
+ |
+ |
+ |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Anthoquions |
- |
- |
- |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Carbohyrate |
+ |
- |
- |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Cumarins |
+ |
- |
- |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
flavonoids |
+ |
- |
+ |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Glycosides |
- |
+ |
+ |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Phenols |
+ |
+ |
+ |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Resins |
- |
+ |
- |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Saponins |
+ |
+ |
- |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Steroids |
+ |
- |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Tannins |
+ |
+ |
+ |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Terpenoids |
- |
+ |
- |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 3 Qualitative analysis of phytochemicals in different solvent extracts of Clausena excavates.
(+)-Presence, (-)- Absence
Quantitative phytochemical screening of different extracts of C. excavateleaf
The total phenolic content was estimated for the hexane, ethyl acetate and methanol extracts of C. excavata and Galic Acid was used as control and the values shown in Table 4. It was observed that 64.24±0.21; 71.16±0.25 and 82.42±0.64mg galic acid equivalent / g dry with were recorded against hexane, ethyl acetate and methanol extracts of C. excavata respectively. Similarly, the total flavonoids were recorded as 57.54±0.87, 62.12±0.64 and 71.28±0.38 mg rutin equivalent / g dry with in hexane, ethyl acetate and methanol extracts of C. excavata respectively (Table 4).
Contents |
Extracts Tested |
Control |
||
|---|---|---|---|---|
Hexane |
Ethyl Acetate |
Methanol |
||
Total Phenolics* |
64.24±0.21 |
71.16±0.25 |
82.42±0.64 |
89.00 ± 0.00 |
Total Flovonaids** |
57.54±0.87 |
62.12±0.64 |
71.28±0.38 |
84.00 ± 0.00 |
Table 4 Estimation of total phenolicsand flavonoids in different extracts of Clausena excavate.
*The total phenolic content was calculated from the calibration curve, and the results were expressed as mg of gallic acid equivalent per g dry weight.
**The total flavonoids content was calculated from a calibration curve, and the result was expressed as mg rutin equivalent per g dry weight.
Values represent mean ± SD of three replications.
There is an inverse relationship between the nutritional intake of antioxidant rich food and the frequency of the human disease Rice Evans et al.20 The ethyl acetate and the methanol leaf extracts of Clausena excavate had shown the gradual increase in a dose dependent manner in the DPPH activity in terms of percentage inhibition depicted in (Figures 1-3). Antioxidant activity (NO2reducing assay) of different solvent extract of C. excavata leaf was assessed and the data pertaining to the experiment were shown that were recorded in the methanol extract of C. excavate was found significant % NO2 reducing activity is directly proportional to the concentration of the extract and also the polarity of the solvents, because, increasing trend of NO2 reducing activity was noticed by the increase in the polarity of the organic solvents. In the present study, the phytochemical present in the solvent extracts also inhibited the free radicals in antioxidant activity method. The Terpenoids have been found to possess antioxidant and antimicrobial properties in various studies.21–23 The cardiac glycosides have been exhibited antioxidant and antimicrobial properties in various plant studies.24–30 Various plant extracts, showed the presence of steroids and these have been inhibited the many bacteria and found to possess antioxidant potentials.31–37 Presence of phenols in extract may explain its potent bioactivities as tannins are known to possess potent antioxidants and antibacterial activities.38–44 The present investigation has shown that methanol extract has active phytochemicals (terpenoids, flavonoids, coumarins and phenols) which are able to inhibit the pathogenic bacteria.
However, the presence of phenolic contents was traditionally speculated with the environmental stress experienced by the plant during the time of sampling. Evaluation of antioxidant properties of plants cannot be carried out accurately by a single universal method, Zengin et al.45 instead a set of assays could able to provide a clue about the activity. Total antioxidant capacity was reported as ascorbic acid equivalents and the method is mainly used for the spectrophotometric quantification of total antioxidant capacity that employs cost effective reagents Prieto et al.46 The strong antioxidant activity was confirmed in methanol extract. Both antimicrobial and antioxidant activity may be due to the strong occurrence of polyphenoloic compound such as terpenoids and phenols. These findings provide scientific evidence to support old-style uses and indicate a promising potential for the development and antimicrobial and antioxidant drug from C. excavate plant.47
The replacement of synthetic with natural antioxidants (because of implications for human health) may be advantageous. In the present study analysis of free radical scavenging activity, total phenolic and flavonoid content of C. excavata clearly showed that it can be used as a potent source of natural antioxidant.
None.
The author declares no conflict of interest.

©2016 Elumalai, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.