MOJ
eISSN: 2573-2919


Research Article Volume 9 Issue 6
Faculty of Chemical Engineering, Benemérita Autonomous University of Puebla, Mexico
Correspondence: José Carlos Mendoza Hernández, Faculty of Chemical Engineering, Benemérita Autonomous University of Puebla, Mexico
Received: October 21, 2024 | Published: December 27, 2024
Citation: Hernández JCM, Reyna SG, Luna ASM, et al. Analysis of the biodegradation of estrogens in water using bacteria. MOJ Eco Environ Sci. 2024;9(6):287-291. DOI: 10.15406/mojes.2024.09.00337
Water contamination by emerging contaminants is a current problem because these contaminants are not eliminated by conventional treatment methods. Among the emerging contaminants we find hormones such as estradiol, which can bioaccumulate and cause alterations between flora and fauna. In this work, the biodegradation process of the algestone-estradiol hormones was effective by the bacteria Pantoea agglomerans NM1 2.1, Escherichia coli KM4 3.2, and Citrobacter freundii KM1 3.1 in Minimal mineral medium, and Consortium 2, formed by Pantoea agglomerans NM2 1.1, Klebsiella pneumoniae KM2 3.2, Pantoea agglomerans NM1 2.1, and Pantoea agglomerans KM1 2.1, enhanced the biodegradation of the estradiol-algestone hormones compared to the individual strains.
Keywords: biodegradation, population growth, estrogen, environment, water
Population growth causes serious pollution problems in surface water bodies that receive discharges of wastewater, which can lead to a low water quality. One of the consequences of population growth is the large quantity of different chemicals that are discharged into wastewater, resulting in increased pollution and environmental damage.1 Contaminants are toxic and persist in the environment for long periods. Many of these contaminants are considered emerging, defined as unregulated chemical substances whose effects may be unknown. These substances are found in concentrations on the order of µg or ng and cause harm to aquatic organisms.2–4 Among these emerging contaminants (EC) are pesticides, herbicides, nanomaterials, phthalates, plastic additives, non-halogenated compounds, personal care products, paraffins, brominated compounds, phytoestrogens, and some pharmaceuticals such as antibiotics, hormones, and analgesics.1
Among the emerging contaminants (EC) are endocrine disruptors, including natural and synthetic estrogens. Natural estrogens are steroid hormones produced primarily by the ovaries and adrenal glands, responsible for the development of the female reproductive system. Synthetic estrogens are used by many women as hormone replacement therapy and contraceptives, and they are the primary source of contamination due to their elimination through excretions, such as feces and urine, which end up in the sewage system. This leads to the feminization of fish and, when they enter human food products, can cause alterations in hormonal levels due to bioaccumulation processes.5
The conventional treatment processes used in wastewater treatment plants are often not sufficiently effective at removing these substances, as they were not designed for that purpose.5 A study conducted by Ayala6 found that some of the emerging contaminants remain at the same concentrations in treated industrial or domestic wastewater.
This is where the challenge of eliminating them arises and preventing their further accumulation in the environment. Therefore, the use of environmental biotechnology systems is proposed, in which, through the metabolic action of bacteria, these contaminants are degraded into simpler products, considering that they use the compounds as the sole source of carbon.
The isolation of bacteria capable of degrading estrogens was carried out using samples obtained from surface water bodies in sites contaminated with these compounds, located in the city of Puebla, Mexico, with coordinates 19°00'16.2" N, 98°12'20.6" W; 18°57'04.4" N, 98°16'12.7" W; 19°01'18.0" N, 98°13'42.5" W; and 19°03'08.5" N, 98°25'43.14" W, according to the Mexican standard PROY-NMX-AA-003-SCFI-2009. The water samples were placed in Luria-Bertani (LB) broth, containing algestona and estradiol at concentrations of 7.5 mg/mL and 0.5 mg/mL, respectively, incubated for 48 hours at 30 °C and 800 rpm. Subsequently, they were isolated on MacConkey, King A, and nutrient agar; their identification was performed through biochemical tests.
In the generation of the inoculum, the strains were propagated in LB broth for 48 hours at 30 °C. Subsequently, they were centrifuged at 11,000 rpm for 10 minutes; the supernatant was separated, and the pellet was resuspended in Minimal Mineral Medium (MMM), adjusted to 0.5 Å at 600 nm, which is approximately equivalent to 1 x 10⁹ CFU/mL.
The determination of biodegradation by the isolated strains was performed in LB broth and MMM containing the estrogens algestona and estradiol at 15 mg/mL and 1 mg/mL, respectively, incubated for 6 days at 30 °C and 800 rpm. After 6 days, the samples were centrifuged at 11,000 rpm for 10 minutes; the supernatant was separated, and readings were taken using an FTIR-ATR spectrophotometer.
The evaluation of biodegradation using two consortia (C1 and C2), each formed by four strains (in the same proportion), was carried out in LB broth and MMM containing estradiol and algestona at concentrations of 1 mg/mL and 15 mg/mL, respectively. The cultures were incubated for 3 days at 30 °C and 80 rpm. Subsequently, the samples were centrifuged at 11,000 rpm for 10 minutes; the supernatant was separated, and readings were taken using an FTIR-ATR spectrophotometer.
The isolated and identified strains belong to the genera Citrobacter freundii, Pantoea agglomerans, Escherichia coli, and Klebsiella pneumoniae, from which the consortia shown in Table 1 were formed.
|
|
Strain |
Gender |
|
Consortium 1 |
NM 1 1.1 |
Citrobacter freundii |
|
KM1 2.1 |
Pantoea agglomerans |
|
|
KM1 3.1 |
Citrobacter freundii |
|
|
KM4 3.2 |
Escherichia coli |
|
|
Consortium 2 |
NM2 1.1 |
Pantoea agglomerans |
|
KM2 3.2 |
Klebsiella pneumoniae |
|
|
NM1 2.1 |
Pantoea agglomerans |
|
|
KM2 2.1 |
Pantoea agglomerans |
Table 1 Bacterial consortia for the biodegradation of estrogens
In Figure 1, the FTIR-ATR spectrum of the hormone algestona-estradiol is shown. In the frequency region of the groups, a band at 3459 cm-1 is observed, which is attributed to the O-H stretching vibration of the -OH groups, followed by the C-H stretching vibration of the aromatic ring (–Ar) at 3049 cm-1. The bands at 2932 cm-1 and 2866 cm-1 correspond to the symmetric and asymmetric C-H stretching of the functional groups -CH2- and -CH3, respectively. The band at 1724 cm-1 was assigned to the vibration of the carbonyl group (C=O) of the algestona. In the fingerprint frequency region, a band at 1447 cm-1 was found, attributed to the C=C vibration of aromatic compounds. The band at 1264 cm-1 corresponds to the Ar-OH vibration of aromatic alcohols, while the bands at 1103 cm-1 and 1206 cm-1 were assigned to the C-C vibration of cycloalkanes. Finally, the band at 705 cm-1 was assigned to the C=C vibration of cycloalkenes.7
The result of the biological treatment of algestona-estradiol in LB broth conducted by the genus Pantoea agglomerans over a period of 3 days is shown in Figure 2. In the spectra corresponding to the strains Pantoea agglomerans KM1 2.1 and NM1 2.1, a similar behavior is observed, with a decrease in the relative intensity of the bands corresponding to the group and fingerprint frequencies described in Figure 1. It can be seen that the bands in the group frequency region for Pantoea agglomerans KM1 2.1 and NM2 1.1 maintain the same shape, indicating that the chemical environment surrounding the molecule is preserved, and the position of the vibration bands does not change. However, in the band at 3459 cm-1, which is assigned to the O-H stretching vibration of the –OH groups, a shift to the left is observed. This suggests that the vibration frequency of the chemical bond in the molecule has decreased, indicating that the chemical structure has weakened or changed.
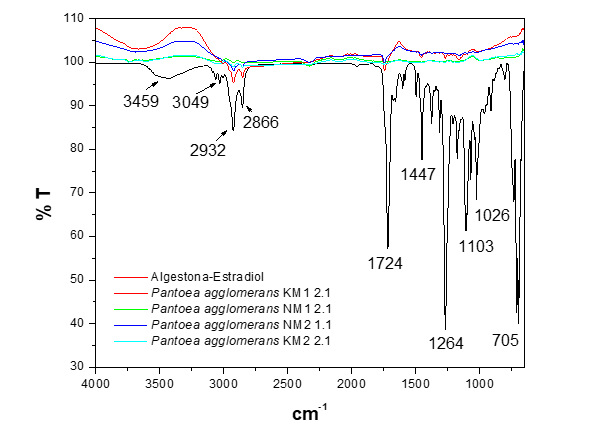
Figure 2 FTIR-ATR spectrum of the biodegradation of the hormone algestona-estradiol by the strains of the genus Pantoea agglomerans in LB broth.
This phenomenon may be due to the molecule exhibiting a lower bond strength, possibly due to interactions such as hydrogen bond formation or changes in the hybridization of the involved atoms, resulting in a compound with a simpler chemical structure. In the spectra of Pantoea agglomerans NM1 2.1 and KM2 2.1, no vibration bands are observed, indicating the biodegradation of the estrogen into basic components such as CO2, water, and inorganic molecules, which are not active in the mid-infrared spectrum for detection.
The biodegradation process of the hormone algestona-estradiol, conducted by the strains Citrobacter freundii NM1 1.1 and KM1 3.1 (Figure 3) in LB broth, shows in the spectrum of Citrobacter freundii NM1 1.1 a leftward shift of the band at 3459 cm-1, attributed to the O-H stretching vibration of the -OH groups. This indicates a change in the chemical structure of the molecule, transforming the hormone into a simpler compound over a period of 72 hours. On the other hand, in the spectrum of Citrobacter freundii KM1 3.1, no characteristic vibration bands of the hormone's chemical structure are observed in the region from 4000 to 650 cm-1, indicating the absence of the characteristic functional groups of the molecule or active groups due to the formation of products such as metabolites resulting from the partial degradation of the hormone algestona-estradiol.
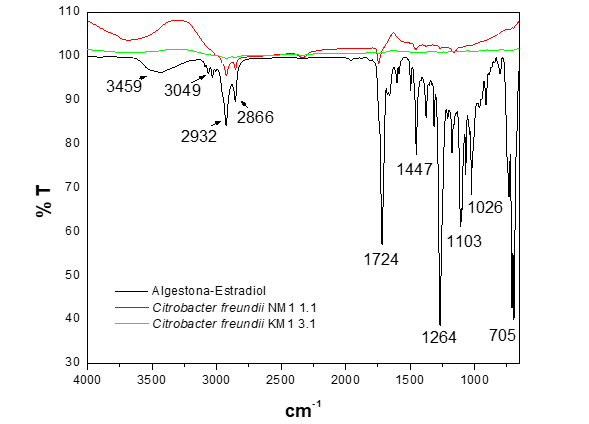
Figure 3 FTIR-ATR spectrum of the biodegradation of the hormone algestona-estradiol by the strains of the genus Citrobacter freundii in LB brothl.
Based on the above, it can be deduced that complete biodegradation occurred, resulting in mineralization.
The analysis of the biodegradation of the hormones at 72 hours, using the strains Klebsiella pneumoniae KM2 3.2 and Escherichia coli KM4 3.2 (Figure 4), shows in the spectrum of the strain Klebsiella pneumoniae KM2 3.2 a similar behavior to the shift in the band at 3459 cm-1, which is attributed to the O-H stretching vibration of the -OH groups, as observed when using Pantoea agglomerans KM1 2.1 and Pantoea agglomerans NM1 2.1 (Figure 2), as well as the strains Citrobacter freundii KM1 1.1 and Citrobacter freundii KM1 3.1 (Figure 3). Furthermore, complete degradation of the hormone algestona-estradiol is observed when using Escherichia coli KM4 3.2 in LB broth.
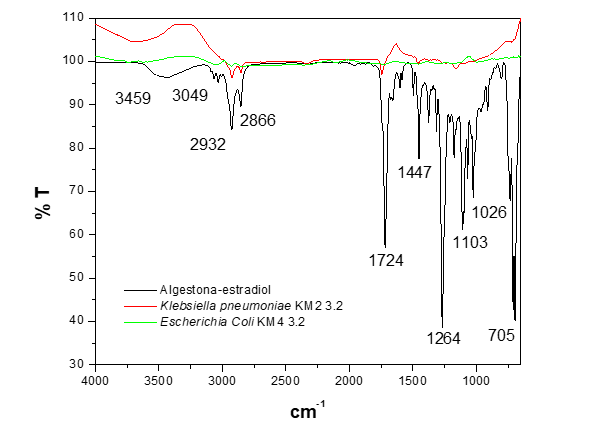
Figure 4 FTIR-ATR spectrum of the biodegradation of the hormone algestona-estradiol by the strains Klebsiella pneumoniae KM2 3.2 and Escherichia coli KM4 3.2 in LB broth.
In the evaluation of the biodegradation of the functional groups present in the hormone algestona-estradiol by various microbial strains of the genus Pantoea agglomerans (Figure 5), a general pattern of biodegradation was observed in all the strains studied. However, a distinctive finding was presented in the spectrum of the strain KM1 2.1, in which the bands of the characteristic functional groups of the algestona-estradiol hormone structure are still observed, albeit with lower intensity, indicating primary biodegradation without reaching the oxidation or mineralization process.
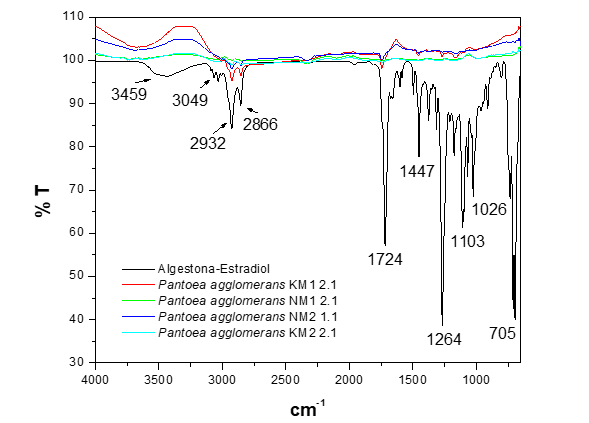
Figure 5 FTIR-ATR spectrum of the biodegradation of the hormone algestona-estradiol by the strains of the genus Pantoea agglomerans in MMM.
In the comparative study of the biodegradation of the functional groups present in the hormone algestona-estradiol (Figure 6), it was observed that the strain of Escherichia coli demonstrated greater efficacy in degrading these components compared to Klebsiella pneumoniae. While Escherichia coli effectively degraded the structure of the hormone algestona-estradiol, Klebsiella pneumoniae showed limitations, as vibration bands of functional groups are still observed in the spectrum.
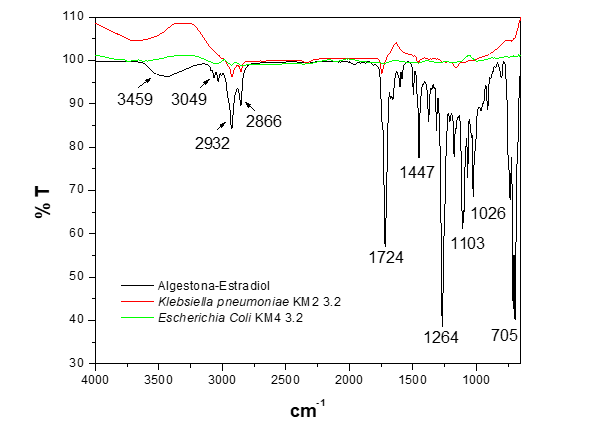
Figure 6 FTIR-ATR spectrum of the biodegradation of the hormone algestona-estradiol by the strains Klebsiella pneumoniae KM2 3.2 and Escherichia coli KM4 3.2 in MMM.
In the study of the biodegradation of the hormone algestona-estradiol using strains of the genus Citrobacter freundii in MMM broth (Figure 7), significant differences were observed in the degradation efficiency among the analyzed strains, as low-intensity bands characteristic of the functional groups were still present. The strain NM1 1.1 did not achieve complete biodegradation of the structure of the hormone algestona-estradiol. On the other hand, the strain KM1 3.1 demonstrated a different capacity, successfully biodegrading the structure to its mineralization.
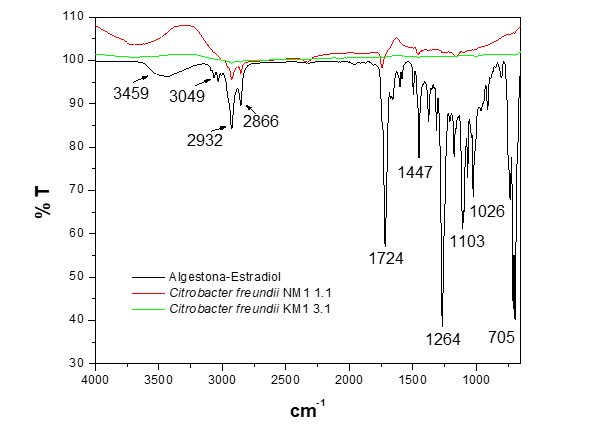
Figure 7 FTIR-ATR spectrum of the biodegradation of the hormone algestona-estradiol by the strains of the genus Citrobacter freundii in MMM broth.
Figure 8 shows the mineralization of the hormone algestona-estradiol by consortia 1 and 2 in LB broth, with the absence of vibration bands of the functional groups of the chemical structure observed.
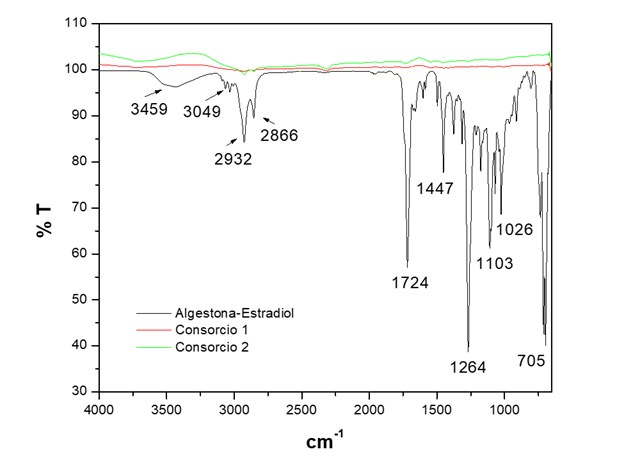
Figure 8 FTIR-ATR spectrum of the biodegradation of the hormone estradiol-algestona by bacterial consortia in LB broth.
In the biodegradation of the hormones estradiol-algestona in MMM (Figure 9), consortium 1 showed the biodegradation of the hormone structures, evidenced by the disappearance and low intensity of the vibration bands of the functional groups. For consortium 2, mineralization is observed due to the absence of vibration bands.
The bacteria isolated in this study, including Citrobacter freundii, Pantoea agglomerans, Escherichia coli, and Klebsiella pneumoniae, showed the ability to biodegrade emerging contaminants such as estradiol under aerobic conditions. This aligns with studies that have demonstrated that various types of microorganisms possess this bioremediation capability for emerging contaminants, such as Rhodococcus sp. RCBS9 (Hao et al., 2024), isolated from a dairy farm, as well as Oleiagrimonas, Pseudomonas, Terrimonas, and Nitratireductor, isolated from mangrove sediments, which degraded estradiol to estrone through aerobic metabolism via dehydrogenation by the enzymatic activity of 17β-hydroxysteroid dehydrogenase, as reported by Zhang et al.8 The bacteria in the present study were able to degrade estradiol into simpler compounds through enzymatic activity, which is consistent with the findings of Bala and colleagues,9 who demonstrated that, through bioremediation techniques, contaminants are degraded and converted into less toxic forms.
The use of bacterial strains and consortia for the removal of endocrine-disrupting compounds from water, such as estrogens, specifically the hormone estradiol-algestone, is effective as a biological treatment. The bacteria can utilize these hormones as their sole carbon source, and together, within a consortium, they exhibit a synergistic effect, as reported by Weber et al.,10 who used a mixed culture for the degradation of estradiol and ethinylestradiol using strains such as Acinetobacter calcoaceticus, Pseudomonas putida, and Comamonas testosteroni.
The removal of estradiol found in this work coincides with that reported by Xiong et al.11 with a biodegradation of 90% of a concentration of 10 mg/L of E2 in 7 days Stenotrophomonas maltophilia SJTH1 using it as the sole carbon source by; Ismanthus et al.12 where the bacterium Cellulosimicrobium funkei degrades 90% of E2; Nawaz et al.13 Laccase enzymes from Bacillus ligniniphilus L1 removed 62%; Prakash and Chaturvedi14 Pseudomonas citronellolis BHUWW1 metabolizes 94% of 10 mg/L E2 after 8 days using sodium acetate as supplementation; Cao et al.15 degraded 80% of E2 at concentrations of 10 mg/L by Sphingobacterium sp. GEMBCS-01; Zhang et al.16 by Ochrobactrum sp strain FJ1 degrades 98 ± 1% estradiol in 10 days and in a very short time compared to what was reported by Estrada et al.,17 where the performance of a submerged membrane bioreactor (SMBR) was evaluated at a pilot plant level to remove estrogens (E1, E2, and EE2) present in the wastewater of the metropolitan area of Mexico City (CDMX). In that study, favorable results were obtained after 94 days of operation, with the best removals achieved at 234 days, which represents a significantly longer time in contrast to the system evaluated in the present research work.
The biodegradation results in both media used in the experiment (MMM and LB broth) were favorable. However, the use of a minimal medium promotes the utilization of hormones as the sole carbon source, as reported by Xiong et al.11 with the bacterium Microbacterium hominis SJTG1, which degraded almost 100% of 10 mg/L of E2 in minimal medium in 6 days. This implies a low-cost and feasible system for large-scale application.
The strains that achieved the best biodegradation of the estradiol-algestone hormone in LB broth were Pantoea agglomerans NM1 2.1 and Citrobacter freundii KM1 3.1, while in MMM, they were Pantoea agglomerans NM1 2.1, Escherichia coli KM4 3.2, and Citrobacter freundii KM1 3.1, using the contaminant as the sole carbon source.
Consortium 2, formed by Pantoea agglomerans NM2 1.1, Klebsiella pneumoniae KM2 3.2, Pantoea agglomerans NM1 2.1, and Pantoea agglomerans KM1 2.1, enhanced the biodegradation of the estradiol-algestone hormones compared to the individual strains, using them as the sole carbon source and mineralizing them. This indicates that the consortium can be employed as a biotechnological process for the removal of these emerging contaminants without generating toxic byproducts.
None.
None.
The authors declare no conflict of interest in writing the manuscript.

©2024 Hernández, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.