MOJ
eISSN: 2573-2919


Mini Review Volume 8 Issue 3
1Department of Environmental Sciences, COMSATS University Islamabad, Abbottabad Campus, Pakistan
2Department of Microbiology, Hazara University Mansehra-21300 KPK, Pakistan
3Department of Microbiology, Abasyn University Peshawar-25120 KPK, Pakistan
4Department of Emergency Medicine, Ayub Medical College, Pakistan
5DHQ Hospital KDA Kohat-26000 KPK, Pakistan
Correspondence: Aleesha Jamshed, Department of Environmental Sciences, COMSATS University Islamabad, Abbottabad Campus, Abbottabad-22022 Pakistan
Received: May 30, 2023 | Published: June 13, 2023
Citation: Jamshed A, Iqbal A, Ali S, et al. A quick review on the applications of nanomaterials as adsorbents. MOJ Eco Environ Sci. 2023;8(3):86-89. DOI: 10.15406/mojes.2023.08.00278
Urbanization and Industrialization have led to release of higher heavy metals amounts into the atmosphere especially aqueous regions. Heavy metals contaminations of potable water have become a serious challenge especially with toxic elements like mercury, lead, zinc, boron and cadmium. Lot of biological constituents has attracted many scientists and researchers due to qualities of cheap and effectiveness for removing heavy metals from waste water. The nanostructured adsorbents exhibit much higher effectiveness and faster rates of adsorption in treatment of water as compared to conservative materials principally because of the remarkably higher surface areas. In the current review, it has been described that the nanomaterials can be used successfully as cost-effective, ecologically friendly, and efficient adsorbents for the elimination of different toxic substances from wastewater.
Keywords: heavy metals pollutions, nanomaterials, adsorbents, wastewater treatment
Water is one of the most vigorous necessities of life for plants, animals and human beings as tremendously cheap commodity next only to air.1 Perhaps as it is very cheap, it faces massive environmental insults from diverse sources including agricultural effluents, domestic wastes, industrial effluents and leachates from solid waste dumps.2 These pollutants impose countless deleterious hazards to human beings particularly, who have to expose to them through both natural and anthropogenic activates like drinking, cooking and washing. Among all these contaminants, heavy metals are most perilous one due to unique attributes like persisting and non-biodegradable to environment. Hence, they cause complex health challenges like cardiovascular and neurological malfunctions, kidney issues, liver damage, epigastria pain, vomiting, nausea, severe diarrhea, skin corrosion, lungs and respiratory track carcinoma.3 The heavy metals comprise mercury, arsenic, cadmium, chromium, lead, Copper, Cobalt, Zinc, Iron, and Nickel.4
These are categorized into three classes; radionuclides (include U, Ra, Th, Am, etc.), toxic metals (include Cr, Hg, Pb, Cu, Sn, Zn, Ni, As, Cd, Co, etc.), precious metals (include Pt, Pd, Ag, Ru, Au, etc. Heavy metals significantly toxic category elements are dangerous for living organisms even in low concentration as they tend to bioaccumulation.5 The comparative intensification of chemical inside the body of organisms more than that of environmental concentration with the passage of time is called bioaccumulation. Heavy metal elements enter in the water resources like lakes, streams, rivers, and ground waters through domestic and industrial wastes. Such kind of heavy metals contain activated carbons and some several ion exchange resins often originates from the industries of pigments, dyes, photography, metal cleanings, galvanometric, mining, electroplating and leathers.6
The wastewater proportion produced in Pakistan is 962,335 million gallons, including 288,326 million gallons from industries and 674,009 million gallons from municipal societies. The overall discharged wastewater in the rivers is 392,511 million gallons, which comprises 75,771 million gallons industrial wastes and 316,740 million gallons municipal wastes. In addition, residential areas add about 73% wastewater and agricultural sector contributed 16% wastewater.7 These facts and figures emphasis on figuring out extra non-conventional resources of water and wastewater treatments has become one of the challenging issues in this land. The industrial and domestic wastewater is discharged either directly in the sewerage system or in the adjacent fields.8
In few recent years, there has been a significant potential for the rectification of environmental issues on account of nanotechnology and nanoscience advancements. The nanostructured adsorbents exhibit much higher effectiveness and faster rates of adsorption in treatment of water as compared to conservative materials principally because of the remarkably higher surface areas. A diversity of efficient, eco-friendly, and low cost nanomaterials with exclusive functionalities have been recommended for prospective applications in detoxification of groundwater, industrial effluents, drinking water, and surface water.9
A perfect adsorbent for the purposes of wastewater treatment should fulfill the following standards:
In the last few years, several studies have demonstrated that the nanomaterials can fulfill most of these requirements.10 It has been verified that the nanomaterials for example carbon nanotubes (CNTs), ferric oxide (Fe3O4), graphene, manganese oxide (MnO2), magnesium oxide (MgO), zinc oxide (ZnO), and titanium oxide (TiO2) can play a significant role in the treatment procedures of waste water. The nanomaterials can be used successfully as cost-effective, ecologically friendly, and efficient adsorbents for the elimination of different toxic substances from wastewater for example azo dyes, heavy metals, etc (Figure 1).11
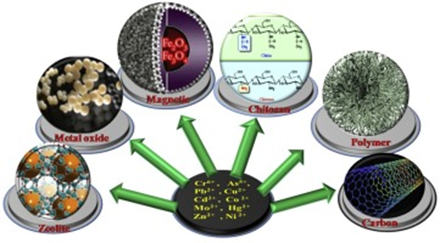
Figure 1 Nanomaterials and heavy metal absorption.12
Phenomenon of adsorption
The adsorption is a surface phenomenon in which the adsorbents accumulate on the surface of adsorbent. When a solution comprising absorbable solutes develop a direct contact with a solid with a structure of extremely porous surface, the intermolecular attractive forces develop between liquid and solid which cause few of the molecules of solute to be deposited or concentrated from the solution onto the solid surface.13 Within different bulky material substances, all the bonding necessities (metallic, covalent, or ionic) of the materials ingredient atoms are occupied by other material atoms. However, the surface atoms of the adsorbent are not surrounded completely by other atoms of adsorbent, thus other adsorbents can also attracted. The particular nature of the bonding is dependent upon the whole information of the involved species, but the process of adsorption is normally categorized as physisorption (the weak van der Waals forces develop among an adsorbent attached to the surface), chemisorption (an adsorbent tied because of electrostatic attraction or with the help of covalent bonds.14
Synthesis of nanoparticles
The nanoparticles can be prepared with the help of different biological and chemical methods. Quite a lot of negating effects have been related to the approaches of chemical synthesis because of the occurrence of various toxic compounds that can be absorbed upon the material surfaces.15 The eco-friendly alternatives to chemical and physical methods are biological systems for the synthesis of nanoparticles through fungus, microorganisms, enzymes, plants and relative extracted compounds. The production of different environmental friendly methods for the synthesis of nanoparticles is emergent into an imperious branch of nanotechnology predominantly silica nanomaterials, which have a number of applications.
Silica nanomaterials with mesoporous characteristics have attracted special consideration from the past few years due to distinctive and versatile physiochemical attributes. In 1992, the Mobil scholars manufactured first silica nanomaterial also called Mobil crystalline materials.16 Silica is one of the most complex and abundant physical families which exist mostly in the form of components of different synthetic products or as minerals. A small number of instances comprise fumed silica, and different sorts of aerogels. The silicon atom shows a tetrahedral organization within an extensive variety of silicates that consist of 4 oxygen atoms surrounded by a central silicon atom. Silica nanomaterials are found to be the in offensive in the course of wastewater treatment and adsorption processes.17
Adsorbent nanomaterial development for wastewater treatment
The most extensively considered nanomaterials for the treatment of wastewater include activated carbons (AC), carbon nanotubes (CNT), graphene,18 MnO2, Fe3O4, Co3O4, MgO, ZnO, TiO2, etc. They sometimes can be prepared in diverse structural forms for example particles, sheets, and tubes.19
Carbon based nanoparticles
The nanoparticles that are entirely made of carbon are categorized into different forms such as carbon nanofibers, carbon black, graphene, carbon nano tubes, and fullerenes. Fullerene (C60) is an imperative nanoparticle based upon carbon having spherical shape and designed by carbon atoms by joining together with the help of sp2 hybridization. Approximately 30-2000 carbon atoms collectively held together in a globular assembly with a diameter ofa range from 8-9 nm of a single layer and around 5-38 nm in the multi-layered fullerene.20 The graphene is an additional allotrope of carbon that exists in two dimensional flat surfaces. The graphene sheet is typically of about 0.5 nm of thickness. Furthermore, a different honeycomb lattice graphene made structure of nanofoils carbon are carbon nanotubes (CN). The identical nanofoils of graphene are functional to produce carbon nanofibers by wounding into cone or cup shapes rather than immovable cylindrical tubes.21 The amorphous morphology intended by carbon, mostly spherical in shape with 30-80 nm in diameter. There are very significant relations between the materials as they bound in clustering shapes and manufactured around 500 nm agglomerates (Figure 2).22
Figure 2 Carbon based nanomaterials for absorption.23
Activated Carbon (AC)
Initially, AC was practiced as adsorbents; but, because of the problems related to removal of heavy metals and dyes at the levels of parts per billion (ppb), CNTs, graphene, and fullerenes were used as nanosorbents for overcoming this trouble. AC characteristically has high surface area, high porosity, and can be designed from voluntarily accessible carbonaceous precursors for example coal, coconut shells, agriculture wastes, and wood. AC is used on a wide scale for the elimination of organic and inorganic pollutants from effluent watercourses and for treatment of waste water. Moreover, it holds a considerably weak character of acidic ion exchange, allowing it to eliminate metal pollutants and to adsorb toxins from wastewater. The adsorption of pentavalent arsenic has been experimentally demonstrated on granular activated carbon (GAC).24
AC prepared by coconut tree along with sawdust as an adsorbent reported to be effective for the elimination of Cr+6 from waste water. The stability and sorption of mercury (Hg) was also reported on AC in terms of controlling emission of gases. Powdered activated carbon (PAC) equipped from Eucalyptus camaldulensis was also reported and presented a sorption capacity of 0.85-0.89m mol g-1 at 60oC, for copper and lead correspondingly. An innovative grafted AC with sodium polyacrylate was reported which use gamma radiation to raise the amount of functional groups on the surface that enlarged the effectiveness of metal ions adsorption by AC. The higher adsorption capability and lower prices make AC auspicious material substances for the removal of heavy metals and dyes.25
Carbon Nanotubes (CNTs)
These nanotubes were developed very first time by Iijima with an exclusive electronic, structural, semiconductor, and optoelectronic, in addition to mechanical, physical, and chemical properties. CNTs have been functional extensively to eliminate dyes and heavy metals during treatment of wastewater. CNTs are found to be one of the most capable sorbents for the treatment of wastewater due to their larger sorption capacities for artificial dyes. Multi-walled carbon nanotubes (MWCNTs) have been revealed to exceed loaded nanowire of cadmium hydroxide with activated carbons in terms of their effective elimination of safranin from wastewater.26 But, only few researches were stated on the applications of CNTs for removal of dyes from waste water. In addition, CNTs were characteristically directly used without additional handling. Thus, the functionalization of CNT has been started to introduce several functional groups which offer innovative adsorption spots. Amongst such adjustments, oxidation is an informal technique for introducing carbonyl and hydroxyl groups to the CNT’s side walls (Figure 3).27
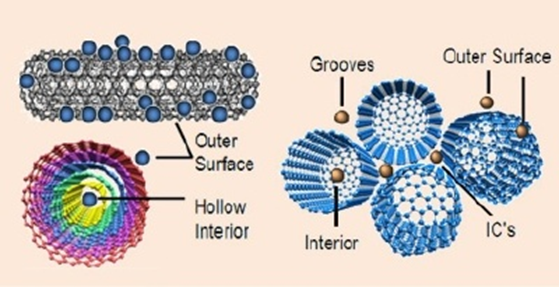
Figure 3 Carbon nanotubes for absorption.28
Graphene
It has been reported useful as nanosorbents, classically comprises of one or more layers of carbon atoms, and holds an exclusive two-dimensional morphology and excellent thermal, electrical, and mechanical characteristics. By means of p–p stacking interactions and Van der Waals’ attractive forces, the sorption of dyes on graphene nanosheets of few layer scan be recognized. For modifying the physical and chemical attributes and improving the engineering of graphene oxide nanosheets (GONSs) by reduced graphene oxide (rGONSs), it can be incorporated firstly into compound material substances.29
Both single GONSs layers and rGONSs layers have larger pelectronic surfaces and higher aspect ratios which offer robust intermolecular forces among adsorbing materials. The rGONSs present evidently rapid adsorption kinetics as compared to CNTs because of the opened-up layer structures. More essentially, rGONSs are much inexpensive as compared to SWCNTs. Amongst diverse carbon based material substances, the MWCNTs, SWCNTs and rGONSs showed superior adsorption abilities for synthetic organic compounds (SOCs, biphenyl, and phenanthrene) in waste water (Figure 4).30

Figure 4 Graphene nanomaterials for absorption.31
Metal oxide-based nanomaterials
Metals or metal oxide-based nanomaterials are further inorganic substances that are used far and wide for the removal of heavy metals, ions and dyes. The metals or metal oxides of nanosize, together with Fe3O4, TiO2, MnO2, MgO, ZnO, and CdO deliver specific affinity and high surface area. Metal oxides hold low solubility, negligible environmental influence, and are not complicated in the formation of secondary contaminations; they have also been accepted as adsorbents to eliminate heavy metal ions and dyes. The one of most prevalent components is iron in the planet Earth.32
The ease of synthesis and predictability of resource reduce ferric oxides of nano size to be less expensive sorbents for toxic metal adsorption. As elemental iron is ecologically friendly, nano size Fe3O4 can be directly pumped to polluted locations with insignificant risks of secondary pollution. Several reports conferred the impact of diverse constraints on the elimination of metal ions by ferric oxides magnetic nanoparticles. Such as, the sorption efficacy of Ni+2, Cd+2, Cr+6 and Cu+2 ions by ferric oxides nanoparticles were strongly dependent upon temperature, pH, quantity of the sorbents and the incubation period.33,34
The occurrence of heavy metals, ions and dyes in wastewater has become a foremost alarm for human health and environment conservation. The process of removing these materials has not extended the optimum settings. On the basis of unique characteristics of nanomaterials, they have been extensively studied for removals of heavy metals and other harmful substances from wastewater because of their higher surface areas and lower particles sizes that made them superior for active adsorption. Adsorption practices by means of nanomaterials are greatly effective, might be easily employed and performed for the elimination of inorganic and organic pollutants. It appears very reasonable that these kinds of adsorbents can find wides preadviable application in the treatment of wastewater in future.
None.
None.
The authors declared that there is no conflict of interest.

©2023 Jamshed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.