MOJ
eISSN: 2573-2919


Plasticity in physiological traits is necessary for the survival and development of woody species in the severe conditions of tropical dry forest. We selected five study sites in a gradient of soil moisture availability, located in dry forest of India. We identified 12 physiological traits (viz., relative water content, RWC; leaf dry matter content, LDMC; specific leaf area, SLA; leaf carbon concentration, LCC; leaf nitrogen concentration, LNC; leaf phosphorus concentration, LPC; chlorophyll concentration, Chl; stomatal conductance, Gsmax; photosynthetic rate, Amax; intrinsic water use efficiency, WUEi; biomass increment, Bio Incr; relative growth rate, RGR), which are considered important for the survival and growth of plant species in tropical dry forest, and measured their range and plasticity in woody species, including trees and shrubs, across the selected study sites. Further, we analysed the response of physiological traits to variations in soil moisture content (SMC) across species as well as across study sites. Across the five study sites, the selected traits exhibited remarkable plasticity, both within as well as among species. The associations of physiological traits with soil properties were also significant. The study shows that all physiological traits under study affect RGR directly or indirectly. However, the strength of effect is determined by environmental parameters, particularly the SMC. Step wise multiple regression indicates that more than 80% variability in RGR can be explained by SLA and WUEi alone. We suggest that for predicting the vulnerability of tropical dry forest communities to changes in climatic conditions, further investigations examining trade-offs among physiological traits and habitat conditions are needed.
Keywords: tropical dry forest, woody species, physiological traits, phenotypic plasticity, soil properties
Phenotypic plasticity has been widely recognized as an important aspect of how organisms develop, function and evolve in their environments.1,2 This biological characteristic corresponds to the ability of an organism to adjust its performance by altering its morphology, physiology and life-history in response to varying environmental conditions.3,4 Physiological plasticity is usually associated with a change in properties brought about by reversible rearrangements of sub-cellular organelles, and represents lower costs and a more rapid response to environmental stress.5 Nevertheless, physiological adjustments may act as a primary signal that could lead to longer-term responses in morphology.6
Tropical dry forests are found in the regions which exhibit remarkable variations in the annual rainfall, length as well as severity of dry seasons, and the mean annual temperature.7,8 Several studies have suggested that plant water availability may be one of the determining factors affecting functional traits, and influencing assemblage of woody species in tropical dry forest.9–19 Woody species in tropical dry forests are subjected to anthropogenic disturbances, and are highly influenced by changes in climatic conditions, therefore it is necessary to understand the factors determining species distribution and growth patterns. This becomes even more urgent in the face of climate change as findings can help to predict the vulnerability of forest communities to climate change.
The study described in this communication was executed in the Vindhyan Highlands situated in Sonebhadra District of Uttar Pradesh, India (21º 29′–25º 11′ N and 78º 15′–84º 15′ E). We selected five study sites (viz., Hathinala, Gaighat, Harnakachar, Ranitali, Kotwa) in a gradient of soil moisture availability. Among the selected sites, Hathinala was most moist and Kotwa was the driest site. For detail information about various aspects of the study region, see Chaturvedi et al.,20,21 Chaturvedi et al.,22 Chaturvedi & Raghubanshi,23 Chaturvedi & Raghubanshi,16 Chaturvedi et al.24,25 On the basis of literature survey on tropical dry forests, we selected 12 physiological traits (viz., relative water content, RWC; leaf dry matter content, LDMC; specific leaf area, SLA; leaf carbon concentration, LCC; leaf nitrogen concentration, LNC; leaf phosphorus concentration, LPC; chlorophyll concentration, Chl; stomatal conductance, Gsmax; photosynthetic rate, Amax; intrinsic water use efficiency, WUEi; biomass increment, Bio Incr; relative growth rate, RGR), which are considered important for the survival and growth of plant species in tropical dry forest, and measured their range in woody species, including trees and shrubs, across the five study sites. Further, we analysed the response of physiological traits to variations in soil moisture content (SMC) across species as well as across study sites. For detail description of study design and the protocol for functional trait measurements, see Chaturvedi26 and Chaturvedi & Raghubanshi.27 Phenotypic plasticity of plant traits for each species across the study sites were calculated following Callahan28 as:
Result of the study showed that among trees, maximum SLA (160cm2 g-1), LNC (2.5%), LPC (0.4%), Chl (2.0 mg g-1), Gsmax (0.34mol m-2 s-1), Amax (15μmol m-2 s-1) and RGR (0.09cm2 cm-2 yr-1) were accounted for Terminalia tomentosa, highest RWC (99%) for Lannea coromandelica, greatest LDMC (38%) for Hardwickia binata, maximum LCC (46%) for Ficus racemosa, and highest WUEi (62μmol mol-1) and Bio Incr (2.1kg mo-1) for Adina cordifolia (Table 1). Among shrubs, maximum RWC (95%), SLA (159cm2 g-1), LNC (2.2%), Chl (1.9mg g-1), Gsmax (0.3mol m-2 s-1), Amax (14μmol m-2 s-1) and Bio Incr (0.11kg mo-1) were reported for Lantana camara, greatest LDMC (37%) and LCC (47%) for Grewia hirsuta, highest LPC (0.3%) for Ziziphus oenoplea, and maximum WUEi (65μmol mol-1) and RGR (0.16cm2 cm-2 yr-1) for Woodfordia fruticosa (Table 2).
Trait |
Min |
Max |
Mean (±S.E.) |
Plasticity (%) |
RWC (%) |
68.6 (Gardenia turgida, HN) |
98.9 (Lannea coromandelica, KT) |
93.1 (±1.0) |
30.6 |
LDMC (%) |
33.0 (Gardenia turgida, HN) |
37.7 (Hardwickia binata, RT) |
35.0 (±0.2) |
12.5 |
SLA (cm2 g-1) |
61.3 (Ficus racemosa, KT) |
159.6 (Terminalia tomentosa, HN) |
120 (±4.6) |
61.6 |
LCC (%) |
42.4 (Lannea coromandelica, HN) |
46.4 (Ficus racemosa, KT) |
44.6 (±0.1) |
8.62 |
LNC (%) |
1.30 (Ziziphus nummularia, HN) |
2.50 (Terminalia tomentosa, RT) |
1.83 (±0.03) |
48 |
LPC (%) |
0.10 (Buchanania lanzan, RT) |
0.40 (Terminalia tomentosa, GG) |
0.22 (±01) |
75 |
Chl (mg g-1) |
0.60 (Buchanania lanzan, RT) |
2.00 (Terminalia tomentosa, HN) |
1.21 (±04) |
70 |
Gsmax (mol m-2 s-1) |
0.20 (Diospyros melanoxylon, KT) |
0.34 (Terminalia tomentosa, HN) |
0.26 (±0.01) |
41.2 |
Amax (µ mol m-2 s-1) |
4.60 (Gardenia turgida, HN) |
15.3 (Terminalia tomentosa, RT) |
11.4 (±0.3) |
69.9 |
WUEi (µ mol mol-1) |
22.9 (Ziziphus glaberrima, HN) |
62.1 (Adina cordifolia, KT) |
46.1 (±1.1) |
63.1 |
Bio Incr (kg mo-1) |
0.20 (Shorea robusta, RT) |
2.10 (Adina cordifolia, HN) |
0.76 (±0.05) |
90.5 |
RGR (cm2 cm-2 yr-1) |
0.005 (Shorea robusta, RT) |
0.09 (Terminalia tomentosa, HN) |
0.04 (±0.003) |
94.4 |
Table 1 Range of physiolological traits for tree species (n = 40) across the five study sites. RWC, relative water content; LDMC, leaf dry matter content; SLA, specific leaf area; LCC, leaf carbon concentration; LNC, leaf nitrogen concentration; LPC, leaf phosphorus concentration; Chl, chlorophyll concentration; Gsmax, stomatal conductance; Amax, photosynthetic rate; WUEi, intrinsic water use efficiency; Bio Incr, biomass increment; RGR, relative growth rate; HN, Hathinala; GG, Gaighat; HK, Harnakachar; RT, Ranitali; KT, Kotwa. Source: Chaturvedi (2010)
We observed that the trait plasticity of physiological traits was greatest for relative growth rate (RGR) (94.4%) among tree species and for biomass increment (Bio Incr) (90.0%) among shrubs. Lowest plasticity among trees was observed for leaf carbon concentration (LCC) (8.62%), and among shrubs for leaf dry matter content (LDMC) (6.54%). Maximum RGR among tree species was estimated for Terminalia tomentosa (0.09cm2 cm-2 yr-1) at Hathinala site, and minimum for Shorea robusta (0.005cm2 cm-2 yr-1) at Ranitali site. Greatest Bio Incr among shrubs was observed for Lantana camara (0.11kg mo-1) at Hathinala site, and lowest in Grewia hirsuta (0.01kg mo-1), also at Hathinala site. Greatest LCC among tree species was observed for Ficus racemosa (46.4%) at Kotwa and lowest for Lannea coromandelica (42.4%) at Hathinala site. Among shrubs, highest LDMC was found for Grewia hirsuta (36.7%) at Ranitali site, and lowest for Ziziphus oenoplea (34.3%) at Hathinala site (Table 1) (Table 2).
Trait |
Min |
Max |
Mean (±S.E.) |
Plasticity (%) |
RWC (%) |
81.8 (Woodfordia fruticosa, RT) |
95.4 (Lantana camara, HN) |
90.1 (±1.5) |
14.3 |
LDMC (%) |
34.3 (Ziziphus oenoplea, HN) |
36.7 (Grewia hirsuta, RT) |
35.7 (±0.2) |
6.54 |
SLA (cm2 g-1) |
72.1 (Grewia hirsuta, RT) |
159 (Lantana camara, HN) |
116 (±11.3) |
54.6 |
LCC (%) |
43.1 (Lantana camara, HN) |
46.8 (Grewia hirsuta, RT) |
45.1 (±0.4) |
7.91 |
LNC (%) |
1.30 (Grewia hirsuta, HN) |
2.20 (Lantana camara, RT) |
1.51 (±0.1) |
40.9 |
LPC (%) |
0.10 (Grewia hirsuta, RT) |
0.30 (Ziziphus oenoplea, HN) |
0.20 (±0.02) |
66.7 |
Chl (mg g-1) |
0.50 (Grewia hirsuta, RT) |
1.90 (Lantana camara, HN) |
0.92 (±0.1) |
73.7 |
Gsmax (mol m-2 s-1) |
0.10 (Grewia hirsuta, RT) |
0.30 (Lantana camara, HN) |
0.18 (±0.02) |
66.7 |
Amax (μ mol m-2 s-1) |
6.00 (Grewia hirsuta, HN) |
13.7 (Lantana camara, HN) |
9.89 (±1.2) |
56.2 |
WUEi (µ mol mol-1) |
45.3 (Ziziphus oenoplea, HN) |
65.2 (Woodfordia fruticosa, RT) |
55.8 (±1.0) |
30.5 |
Bio Incr (kg mo-1) |
0.01 (Grewia hirsuta, HN) |
0.11 (Lantana camara, HN) |
0.06 (±0.01) |
90.9 |
RGR (cm2 cm-2 yr-1) |
0.05 (Grewia hirsuta, HN) |
0.16 (Woodfordia fruticosa, RT) |
0.10 (±0.01) |
68.8 |
Table 2 Range of physiolological traits for shrub species (n = 4) across the five study sites. RWC, relative water content; LDMC, leaf dry matter content; SLA, specific leaf area; LCC, leaf carbon concentration; LNC, leaf nitrogen concentration; LPC, leaf phosphorus concentration; Chl, chlorophyll concentration; Gsmax, stomatal conductance; Amax, photosynthetic rate; WUEi, intrinsic water use efficiency; Bio Incr, biomass increment; RGR, relative growth rate; HN, Hathinala; GG, Gaighat; HK, Harnakachar; RT, Ranitali; KT, Kotwa. Source: Chaturvedi (2010)
The relationship of leaf relative water content (RWC) was strongest with SMC (R = 0.95, P < 0.01). It has been observed that among various soil properties, RWC was positively related with SMC, C, N, P and clay, however, it was negatively related with BD, pH and sand (Figure 1). LDMC also showed strongest relationship with SMC (R = -0.96, P < 0.01). Its relationship with SMC, N, P and clay were negative but the relationship with BD, pH, C and sand were positive. SLA was strongly related with SMC (R = 0.99, P < 0.01) and clay content (R = 0.99, P < 0.01). Its relationship with SMC and clay were positive, whereas, the relationship with BD, pH and sand were negative. LCC showed strongest relationship with clay (R = -0.99, P < 0.01) and it was detected that its relationship with SMC, C, N, P and clay were negative and that with BD, pH and sand were positive. The relationship of leaf nitrogen concentration (LNC) was strongest with pH (R = 0.91, P < 0.05). It was observed that the relationship of LNC with SMC, C, N, P and clay were negative and the relationship with BD, pH and sand were positive. Leaf phosphorus concentration (LPC) showed strongest relationship with clay (R = 0.97, P < 0.01). Its relationship with SMC, C, N, P and clay were positive and the relationship with BD, pH and sand were negative. The strength of relationship of leaf chlorophyll concentration (Chl) was strongest with clay (R = 1.00, P < 0.01). It was observed that the relationship of Chl with SMC, C, N, P and clay were positive, whereas, the relationship with BD, pH and sand were negative. Stomatal conductance (Gsmax) also showed strongest relationship with clay (R = 0.97, P < 0.01). Its relationship with SMC, C, N, P and clay were positive and the relationship with BD, pH and sand were negative. The relationship of leaf intrinsic water use efficiency (WUEi) with clay was strongest (R = -0.98, P < 0.01) as compared to its relationships with other soil properties. It showed positive correlations with BD, pH, N and sand (Figure 1).
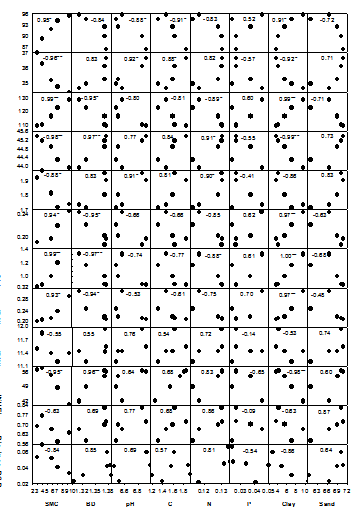
Figure 1 Relationships of physiological traits with soil properties across the five study sites. RWC, relative water content; LDMC, leaf dry matter content; SLA, specific leaf area; LCC, leaf carbon concentration; LNC, leaf nitrogen concentration; LPC, leaf phosphorus concentration; Chl, chlorophyll concentration; Gsmax, stomatal conductance; Amax, photosynthetic rate; WUEi, intrinsic water use efficiency; Bio Incr, biomass increment; RGR, relative growth rate; SMC, soil moisture content; BD, bulk density; C, organic carbon content; N, total nitrogen content; P, total phosphorus content; *P < 0.05; **P < 0.01. Numbers indicate R values. Source: Chaturvedi (2010).
The study showed a wide range of phenotypic plasticity in the woody species of the study sites. They also showed very high variation in their relationships with SMC and other soil physico-chemical properties. These traits work in association for better optimization of photosynthesis. According to Santiago et al.29 the rate of photosynthesis tends to decrease with increasing leaf life-span. It also decreases with increasing tree age and size.30 A positive correlation with stomatal conductance and SLA has been observed by Niinemets et al.31 and is shown to be influenced by structure, LNC, stomatal conductance and carboxylation capacity of leaf.30 In our study, we observed that SLA (R = 0.59, P < 0.001), LNC (R = 0.54, P < 0.001), LPC (R = 0.35, P < 0.001), Chl (R = 0.40, P < 0.001), Gsmax (R = 0.52, P < 0.001), WUEi (R = 0.20, P < 0.05) and Bio Incr (R = 0.24, P < 0.01) showed significant positive association with Amax.
We found that RWC, SLA, LPC, Chl and Gsmax were positively related with SMC, C, N, P and clay, whereas, LDMC, LCC, LNC, Amax and WUEi showed positive association with BD, pH and sand. Therefore, we can interpret that RWC, SLA, LPC, Chl and Gsmax are the important physiological traits for the plant species growing in moist habitats with high stem density, while LDMC, LCC, LNC, Amax and WUEi are important for the plant species growing in dry habitats.
Most of the plant species at our study sites are light demanding and highly deciduous. According to Keeling et al.32 Bio Incr of tree species is affected by functional traits. Physiological and morphological trade-offs imply that shade-tolerant species maximise Bio Incr in the shade due to lower whole-plant light compensation points. According to Keeling et al.32 light demanding species typically have faster foliage turnover, which acts as a significant biomass “drain”, and therefore, greater biomass production is necessary to maintain sufficient leaf biomass.33
The study shows that all physiological traits under study affect RGR directly or indirectly. However, the strength of their effect is determined by environmental parameters and in case of tropical dry forest; soil water availability is the important parameter. Step wise multiple regression indicates that more than 80% variability in RGR can be explained by SLA and WUEi alone. These variables represent the water use economy of a species, and both are also significantly modulated by soil moisture availability. Important point to note here is that Amax is not an important parameter to determine RGR in tropical dry forest, where water economy and extended period of leaflessness are critical. We suggest that for predicting the vulnerability of tropical dry forest communities to changes in climatic conditions, further investigations examining trade-offs among physiological traits of woody species and habitat conditions are needed.
RKC thanks Council of Scientific and Industrial Research, India (award no. 09/13(452)/2012-EMR-I) and Natural Science Foundation of China (NSFC), Chinese Academy of Science, China (grant No. 31750110466) for financial support. RB is thankful to UGC (BSR/BL/17-18/0067) for providing Dr. DS Kothari fellowship for Post Doctoral Research.
Author declares there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.