MOJ
eISSN: 2381-179X


Case Report Volume 9 Issue 1
1Universidad Alfonso X el Sabio, Spain
2Hospital de Día Pio XII, Spain
3Departamento de Investigación, Hospital Universitario Ramón y Cajal, Spain
4Departamento de Estructura y Función de Proteínas, Centro de Investigaciones Biológicas (CSIC), Spain
Correspondence: edro Cuevas, Facultad de Medicina, Universidad Alfonso X el Sabio, 28691-Villanueva de la Cañada, Spain
Received: December 29, 2018 | Published: January 10, 2019
Citation: Cuevas P, Outeiriño L, Ramos J, et al. Single intravitreal etamsylate injection for the treatment of choroidal neovascular membrane formation in neovascular age-related macular degeneration. MOJ Clin Med Case Rep. 2019;9(1):1-3. DOI: 10.15406/mojcr.2019.09.00289
We describe a case of a man presenting an acute visual deterioration in his right eye. Fundus examination identified a submacular haemorrhage with suggestive associated choroidal neovascular membrane. He was treated with a single intravitreal injection of etamsylate. Fundoscopy and optical coherence tomography at the last follow-up visit depicted total clearance of submacular haemorrhage and resolution of choroidal neovascular membrane. These morphological findings were associated with a significant improvement in his visual acuity.
Keywords neovascular age-related macular degeneration, submacular haemorrhage, choroidal neovascular membrane, fibroblast growth factor, intravitreal etamsylate injection
Submacular haemorrhage (SMH) is an accumulation of blood between the neurosensory retina and retinal pigment epithelium (RPE), arising from the choroidal circulation within the macula. It is considered that it usually occurs in the context of neovascular (wet) age-related macular degeneration (ARMD).1 Owing to iron toxicity, SMH damages the RPE and the photoreceptors, affects the fibrin network contraction and causes a subsequent reduced nutrient flux from choriocapillaris ensued of scarring development.2–4 While SMH is not common, wet ARMD patients with coagulopathies to which anticoagulant medication is administered are, nevertheless, particularly susceptible to developing this disease.5 The visual outcome of wet ARMD patients with SMH is typically poor, particularly in eyes with thick blood clots or involving large areas of the macula, and which develop a choroidal neovascular membrane (CNVM).6 Average time for disappearance of the SMH is 6 months.4 Experimental studies support prompt treatment of SMH, as tissue damage occurs within 24hours. Without treatment the natural history of SMH is poor. Search for a safe and effective treatment for removing the blood beneath the macula to hasten visual recovery and prevent irreversible damage to the outer retina is a medical need. There is no standard treatment for acute SMH. Etamsylate is a newly identified therapeutically relevant molecule that could be used in pathological conditions involving aberrant fibroblast growth factor (FGF) signalling7,8 (NOSOTROS). Immunoreactivity for FGF has been reported in CNVM removed surgically from humans with ARMD, which suggests a role of this growth factor in the origin and progression of the disease.9 Furthermore, damage of RPE as it occurs in ARMD, causes the release of FGF which, in turn, could contribute to formation of CNVM by itself.10 Thus, local inhibition of FGF would sum an adequate strategy for resolving CNVM. We describe here the efficacy of intravitreal etamsylate administration in a patient with SMH and CNVM associated with wet ARMD.
The medical history of the patient (a 73 years old man with acute vision loss in his right eye -mere light perception-) was positive for quiescent ARMD (dry form in left eye and wet form in right eye). He was using anticoagulant therapy. Ophthalmologic examination, at baseline and at the follow-up visits after treatment, included best-corrected visual acuity (BCVA) by means of Snellen chart (described in decimal scale), slit-lamp biomicroscopy and colour fundoscopy. At the last follow-up visit optical coherence tomography (OCT) was also performed.
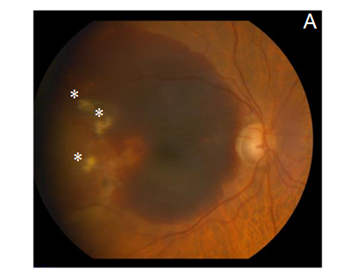
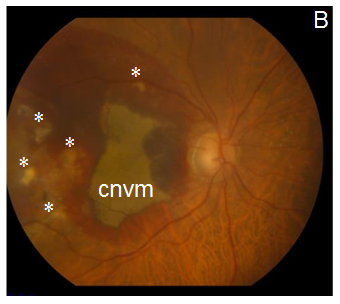
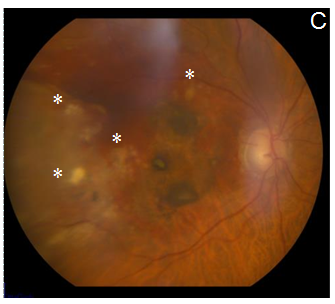
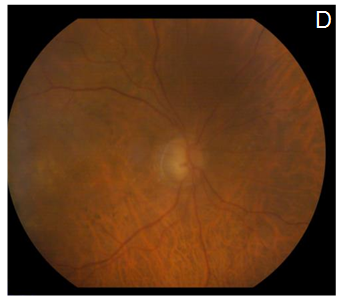
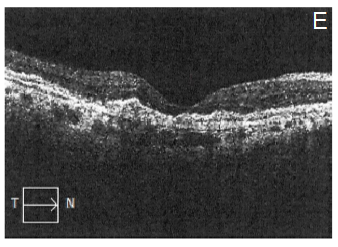
After explaining the nature of the disease, its natural course, and the alternative treatment modalities, the patient gave informed consent to this novel treatment approach. Topical antibiotics were administrated 4 times a day for 1 day before the treatment, and 5 days after it. The procedure was performed under sterile conditions in the operating room. The patient received an intravitreal injection (100µL) of etamsylate (Dycinone®, Sanofi-Aventis, Paris, France) according to the international guidelines for intravitreal injections.11 No ocular side effects were observed upon administration of etamsylate or along the following days. The intraocular pressure remained normal. At baseline, anterior slit-lamp examination was unremarkable. Posterior segment fundoscopy was striking for SMH: it had a thickness capable of inducing significant elevation of the neurosensory retina, which completely obscured the underlying choroidal pattern at biomicroscopy examination (Figure 1A). Two weeks after the treatment, colour fundoscopy revealed a partial resolution of SMH that allowed to visualize a CNVM (Figure 1B) which became imperceptible 4 weeks after treatment (Figure 1C). Furthermore, at the last post-treatment visit (8 weeks) fundoscopy revealed a complete clearance of blood and resolution of soft drusen (Figure 1D), which suggests remodelling and regeneration after the etamsylate treatment. Furthermore, corresponding OCT scans showed great improvement with absorption of the haemorrhage. Central macular thickness (CMT) was 277µm (Figure 1E), and visual acuity became 0.40.
The main point of this study is that the treatment of SHM and CNVM due to ARMD with a single intravitreal injection of etamsylate improves visual acuity. Etamsylate is a drug with a long history of clinical safety that was recently rediscovered as a fibroblast growth factor (FGF) inhibitor.12 FGF was the first inductor of angiogenesis (the formation of new vessels from pre-existing ones) and vascular permeability that was described.13 In haemorrhagic conditions, extravasated blood cells, such as monocyte-derived macrophages, synthesize FGF.14 It has been reported that high levels of FGF induce structural changes in intestinal vessels, resulting in the development of lethal intestinal haemorrhage15 We have previously shown that neovascular growth with its subsequent bleeding can be cancelled by inhibiting FGF.16
Additionally, the haemostatic activity of etamsylate17 may also be involved in the ameliorating effects reported here. Furthermore, this case report seems in full agreement with the use of etamsylate for the treatment of periventricular haemorrhage in infants with very low birth weight.18
Intravitreal injection of etamsylate results in visual improvement in a patient with submacular haemorrhage and choroidal neovascular membrane secondary to neovascular age-related macular degeneration which may otherwise have a poor visual outcome. Obviously, further clinical trials are required to determine with the adequate accuracy the actual extent of the efficacy of the intravitreal administration of etamsylate, in patients presenting submacular haemorrhage and choroidal neovascular membrane in age-related macular degeneration condition.
We thank patient for participating in this study.
The author declares no conflicts of interest.

©2019 Cuevas, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.