MOJ
eISSN: 2574-819X


Short Communication Volume 1 Issue 5
1Departement de Chimie, Universite Cheikh Anta Diop de Dakar, Senegal
1Departamento de Quimica Organica, University of Vigo, Spain
Correspondence: Yagamare Fall, Departamento de Química Orgánica, Facultad de Química and Instituto de Investigación Sanitaria Galicia Sur (IISGS), University of Vigo, Campus Lagoas de Marcosende, 36310 Vigo, Spain, Tel 34 986812320
Co-correspondence: Alioune Fall, Laboratoire de Chimie de Coordination Organique (LCCO), Département de Chimie, Faculté des Sciences et Techniques, Université Cheikh Anta Diop de Dakar, Sénégal, Tel 772644472
Received: October 31, 2017 | Published: November 14, 2017
Citation: Fall A, Diouf O, Gaye M, et al. The furan approach to the synthesis of natural products having highly substituted cyclic ether moieties. MOJ Biorg Org Chem. 2017;1(5):184-187. DOI: 10.15406/mojboc.2017.01.00033
We describe the synthesis of useful building blocks towards the synthesis of many natural products bearing substituted cyclic ether moieties, using our previously described method we called ‘The Furan Approach” and which is based on singlet oxygen oxidation of a furan ring followed by an intramolecular oxa-Michael addition.
Keywords: oxacyclic compounds, furan, singlet oxygen, natural products, stereoselective synthesis
Highly substituted cyclic ethers occur in many natural products exhibiting important biological activities.1-6 These units can be found in monocyclic as well as in polycyclic structures some of them are depicted in Figure 1. Due to the biological importance of these compounds, they have been the targets of numerous synthetic studies.7-13 In our research group we have developed a new methodology for the synthesis of oxacyclic compounds using furan as starting material.14-27 The scope and limitations of this very powerful methodology are being determined.
We now report that using our method we can easily access the 2,6-disubstituted tetrahydropyran and 2,5-disubstituted tetrahydrofuran systems, which are advanced model intermediates towards the synthesis of the natural compounds depicted in Figure 1. It was anticipated that butanetriol (1) could be a common starting material for accessing the 2,6-disubstituted tetrahydropyran 13 (Figure 2) and 2,5-disubstituted tetrahydrofuran 22 (Figure 3).
Thus protection of the C1, C2-hydroxyl groups of 1 with cyclohexanone afforded alcohol 228 (97%) easily converted into iodide 328 in 93% yield. Lithiation of furan 4 and reaction with 3 afforded the alkylated furan 528 (91%). Removal of the cyclohexylidene group of 5 using Dowex 50W-X8 in methanol,29 gave diol 6 28 in 89% yield. The hydroxyl groups of 6 were protected as silylethers affording furan 7.28 Oxidation of 7 with singlet oxygen followed by treatment with acetic anhydride in pyridine, afforded butenolide 828 in 97% yields (2 steps). Treatment of 8 with TBAF led to lactones 928 and 1028 in 35 and 45% yield respectively.
Protection of the hydroxyl group of 9 followed by opening of the lactone ring afforded tetrahydropyran 1328 possible synthon towards the synthesis of decarestrictine L or mucocin (Figure 1). Having demonstrated the feasibility of highly substituted tetrahydropyrans from butanetriol (1), we now turned our attention to synthesizing tetrahydrofurans from the same starting material. In order to apply our method, we needed to prepare furan 19 (Figure 3) which has a side chain one carbon shorter than its homologue30,31 (Figure 2). Swern oxidation of alcohol 2 afforded aldehyde 1428 in 99% yields.
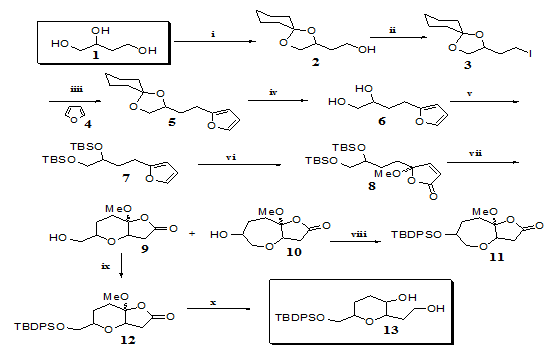
Figure 2 Reagents and conditions:
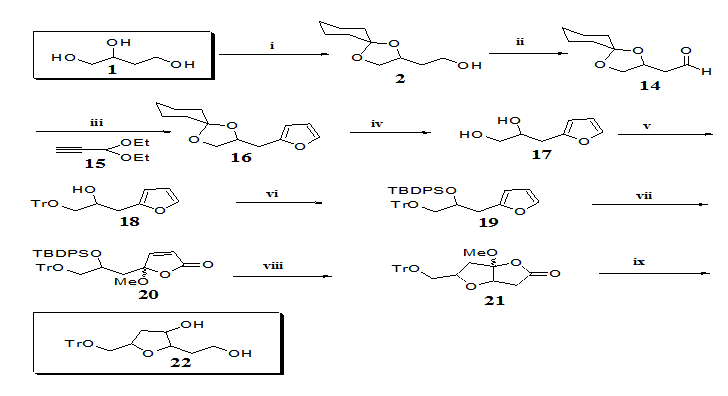
Figure 3 Reagents and conditions:
Treatment of aldehyde 14 with the lithium derivative of alkyne 15 gave a mixture of epimeric propynyl alcohols which were hydrogenated over lindlar catalyst30,31 to provide a mixture of diastereoisomeric (Z)-alkenes which upon treatment with catalytic pyridinium toluene -p- sulfonate (PPTS) gave the desired furan ring in 66% overall yield from the aldehyde 14. Removal of the cyclohexylidene group of 1628 using Dowex 50W-X8 in methanol29 gave diol 1728 in 80% yield. The primary hydroxyl group of 17 was selectively protected as trityl ether and the secondary hydroxyl group as silylether affording furan 19.29 The stage was now set for the oxidation of 19 with singlet oxygen followed by treatment with acetic anhydride in pyridine which afforded butenolide 2028 in 77% overall yield. Treatment of 20 with TBAF led to lactone 2128 in 98% yields. Finally, opening of lactone 21 with LAH afforded tetrahydrofuran 22.28 Compound 22 can be considered as building block towards the synthesis of the natural products bearing a THF moiety depicted in Figure 1.
In conclusion, we have demonstrated that using racemic butanetriol we can apply the “Furan approach “to the synthesis of useful building blocks towards the synthesis of many natural products. As both enantiomers of butanetriol are commercially available, work is now in progress towards the enantioselective synthesis of the natural products described in Figure 1.
The work of the NMR and MS divisions of the research support services of the University of Vigo (CACTI) is gratefully acknowledged. Alioune Fall, Ousmane Diouf, Abdou Salam Sall and Mohamed Gaye thank the University Cheikh Anta Diop (Dakar) for financial support.
The author declares no conflict of interest.

©2017 Fall, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.