MOJ
eISSN: 2574-819X


Review Article Volume 3 Issue 1
1Chemistry Center, Venezuelan Institute of Scientific Research (IVIC), Venezuela
2Deptartment of Chemical Engineering, Central University of Ecuador, Ecuador
3Technological Develop Unit (UDT), University of Concepcion, Chile
Correspondence: Ajoy K Banerjee, Centro de Química, IVIC, Apartado- 21827, Caracas-1020A, Venezuela, Tel 5802 1250 4132 4, Fax 5802 1250 4135 0
Received: November 06, 2017 | Published: January 21, 2019
Citation: Banerjee AK, Maldonado A, Arrieche DA, et al. Boron trifluoride etherate in organic synthesis. MOJ Biorg Org Chem. 2019;3(1):1-9. DOI: 10.15406/mojboc.2019.03.00090
The Lewis acid boron trifluoride etherate has played an important role in organic synthesis. To accomplish the hydroxylation of double bond, cleavage of epoxides, esterification of acids and many cyclization reaction boron trifluoride etherate proved very useful compared to other Lewis acids. These organic reactions are frequently employed to achieve the synthesis of natural products. In addition, boron trifluoride etherate has also been utilized to realize ketalization, thioketalization, alkylation and many others organic reactions.
Keywords: boron trifluoride etherate, hydroboration-oxidation, epoxides, esterification, thioketalization, cyclization
The innumerable application of boron trifluoride etherate in organic synthesis1,2 encouraged us to write a micro review on this reagent. The commercially available boron trifluoride etherate (b.p. 126oC), a brown liquid, is very toxic by inhalation, causes severe burns, reacts violently with water, hot alkali or alkaline earth (not Mg) metals with incandescence. It can be purified by distillation. This review would discuss only four principal use of boron trifluoride etherate: (a) hydroboration–oxidation reaction, (b) cleavage and rearrangement of epoxides, (c) esterification (d) cyclization. In addition, some other minor uses of boron trifluoride etherate will be briefly described.
Hydroboration–oxidation reaction
The cis addition of diborane to an alkene bond provides an extremely useful method of hydration. Diborane can be generated by the addition of sodium borohydride to boron trifluoride etherate in tetrahydrofuran or ether at 0o-5oC. Diborane is the dimer of borane (BH3) and is stable form of this reagent (Scheme1).
The addition of diborane to the alkene is extremely rapid and generally, the reagent adds from the less hindered of the two faces of the π system. The cis addition has been rationalized by a four center transition state. The borane complex resulting from the addition of diborane to an alkene is converted, with retention of stereochemistry, to an alcohol by treatment with basic hydrogen peroxide. Thus 1-methylcyclohexene 1 on hydroboration-oxidation leads the formation of trans-2-methylcyclo-hexanol 2. The mechanistic path has been depicted in (Scheme 2). The method for the conversion of alkene to alcohol by hydroboration-oxidation has been applied for the synthesis of many natural products. Few examples are illustrated below.
Synthesis of (±) junenol and (±) acalomone
The use of hydroboration-oxidation reaction was observed by Banerjee and coworkers during the synthesis3 of eudesmone sesquiterpenes (±) junenol and (±) acalomone. In order to achieve the synthesis of these sesquiterpenes the alkene 3, was selected as starting material and subjected to hydroboration-oxidation to yield the alcohol 4 (Scheme 3). Ketone 5, obtained by the oxidation of the alcohol with Jones reagent4 was made to react with diethyl carbonate. The resulting product was treated with methyl lithium to obtain the ketol 6 whose conversion to the isopropyl ketone 7 was effected by dehydration and hydrogenation respectively. Metal hydride reduction of the ketone followed by oxidation with lead tetraacetate5 in cyclohexane afforded the cyclic ether 8, which was converted to ketoacid 9 by oxidation with chromic acid and acetic acid. Decarboxylation with lead tetraacetate in benzene and pyridine followed by purification over 10% AgNO3 impregnated silica gel afforded (±) acolamone 10. Reduction of acolamone 10 with sodium borohydride in methanol, followed by sublimation of the resulting product yielded junenol 11.
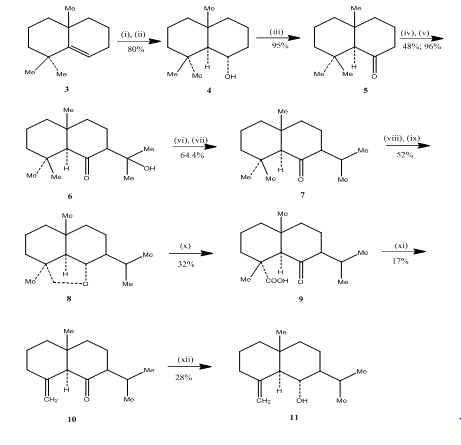
Figure 3 Synthesis of eudesmone sesquiterpenes (±) junenol and (±)-acalomone
Reagents: (i) BF3.Et2O, NaBH4, THF, 0-5°C; (ii) NaOH (10%), H2O2(30%); (iii) CrO3/HMPT; (iv) NaH, CO(OEt)2, DME; (v) MeLi, Et2O, reflux, 2h; (vi) HCl(conc), MeOH; (vii) H2, PtO2, MeOH; (viii) Na, EtOH, reflux; (ix) Pb(OAc)4, C6H12; (x) CrO3, AcOH; (xi) Pb(OAc)4, C6H6, Py, reflux; (xii) NaBH4, EtOH.
Synthesis of pisiferic acid
The use of hydroboration-oxidation has been recorded during the synthesis of pisiferic acid,6 a tricyclic diterpene which shows antibacterial activities against all gram-positive bacteria tested.7 The synthetic route has been depicted in Scheme 4. Hydroboration-oxidation of the alkene 13, prepared from the known8 ketoalcohol 12, was oxidized with jones reagent4 and reduced respectively with metal hydride to give alcohol 14. Oxidation with lead tetraacetate in benzene with 250W tungsten lamp gave the cyclic ether 15. The cleavage of the cyclic ether with zinc, zinc iodide and acetic acid8 furnished pisiferol 16. The transformation of the pisiferol to the ester 17 was achieved in six steps:
The ester l7 was converted into pisiferic acid 18 by heating with aluminium bromide and ethane thiol.
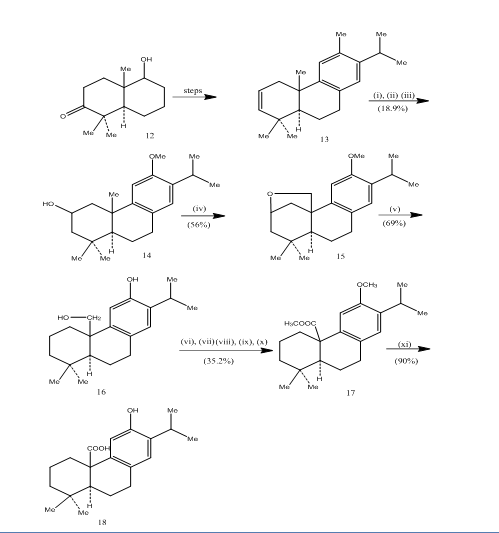
Figure 4 Synthesis of pisiferic acid 18
Reagents: (i) BF3.Et2O, NaBH4; (ii) NaOH (10%), H2O2 (30%), H2SO4-HCrO4; (iii) LiAlH4, THF; (iv) Pb(OAc)4, CaCO3, C6H6, 250w tungsten lamp; (v) Zn, ZnI, MeCOOH; (vi) MeSO4, Me2CO; (vii) H2SO4-HCrO4; (viii) CH2N2, Et2O; (ix) NaBH4, MeOH; (x) TsCl, Py; (xi) NaI, Zn dust, DMF; (xii) AlBr3, (CH2SH)2.
The hydroboration-oxidation reaction has been applied for the synthesis of (±) eudes-4(14),7(11)-diene-8-one,9 taxodione,10 norditerpene alcohols11 and many other terpenes.12 These examples clearly indicate the use of boron tifluoride etherate in the conversion of the alkenes into alcohols and subsequently their transformations into the terpenoid compounds.
Cleavage of epoxides
The epoxides can be cleaved by several reagents. The Lewis acid borontrifluoride etherate has also been used for the cleavage of epoxides and in many cases the resulting product rearranges to ketone. The cleavage of epoxides is also accompanied by cyclization. In this review the cleavage of some epoxides with boron trifluoride etherate and the use of the resulting products in the synthesis of natural products have been discussed.
Synthesis of 6-methoxy-2-tetralone
The cleavage of epoxide with boron trifluoride etherate has been utilized13 for the synthesis of 6-methoxy-2-tetralone 20 (Scheme 5), an important selected starting material for the synthesis of many organic compounds. Epoxidation of the alkene13 19 followed by treatment of the crude product in dichloromethane with boron trifluoride etherate afforded the tetralone 20 in 36% yield. When the cleavage was tried with sulfuric acid the yield of the teralone 20 was improved (39%) along with the formation of other secondary products and thus the chromatographic purification was very laborious.
Synthesis of cuprane
The rearrangement of epoxides by boron trifluoride etherate proved very useful during the synthesis14 of sesquiterpene cuprane. The synthetic route is described in Scheme 6. 6,6-dimethyl-1-p-tolylcyclohexene 21 on epoxidation afforded the epoxide 22 in good yield which on treatment with boron trifluoride etherate in benzene yielded the aldehyde 23 in low yield. The semicarbazone of the aldehyde was heated with potassium hydroxide to furnish the sesquiterpene cuprane 24 in acceptable yield. The synthesis is attractive due to its brevity in steps. The conditions used for the rearrangement of the epoxide 22 are critical because it has the tendency to undergo further rearrangement to the ketone 25.
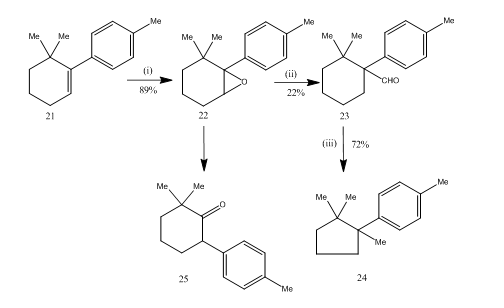
Figure 6 Synthesis of cuprane
Reagents: (i) PhCO3H, CHCl3; (ii) C6H6, BF3Et2O; (iii) NH2NHCONH2, KOH
Synthesis of (±) karahana ether
Boron trifluoride etherate was also used for the cleavage of epoxide during the synthesis15 of karahana ether, a volatile monoterpene which was isolated16 from Japanese hops. The synthetic route is described in Scheme 7. The epoxide 27, obtained from the diene 26, on being treated with boron trifluoride etherate underwent cyclization yielding the product 28. The cyclization probably occurred through the intermediate 27 (i). Metal hydride reduction afforded diol which on tosylation yielded karahanaether 29. The yield is unspecified. The cleavage of epoxides have been utilized for the synthesis of many terpenes like rosenolactone,17 cyperolone,18 maritimol.19
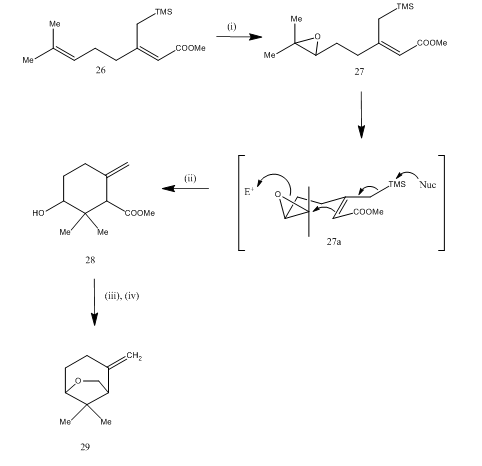
Figure 7 Synthesis of (±) Karahana ether
Reagents: (i) MCPBA; (ii) BF3Et2O; (iii) LiAlH4; (iv) TsCl, Py
Esterification
Esterification is a frequently used reaction for the synthesis of many organic compounds. Boron trifluoride etherate –alcohol is a very convenient reagent for the esterification of many p-amino benzoic acids, aromatic, heterocyclic and unsaturated acids.20 In some esterification reactions the use of this reagent provided superior yield compared with other reagents. Some examples are given in Scheme 8. The acids 30-32 were converted to the esters 33-35 respectively in high yield on treatment with boron trifluoride etherate-alcohol reagent. Marshall and collaborators21 used the same reagent for the esterification of carboxylic acids. Dymicky22 prepared several formats in high yield from formic acid and alcohol in the presence of a catalytic amount of boron trifluoride -methanol complex. The other catalysts e.g. sulfuric acid, p-toluene sulfonic acid was not so efficient like boron trifluoride- methanol complex.
Jackson and collaborators23 have developed an efficient method for the conversion of alcohols 37-39 and acids 40-42 directly to the corresponding t-butyl derivatives in good yield using t-butyl trichloroacetimidate 36 in the presence of a catalytic amount of boron trifluoride etherate as exhibited in Scheme 9. This method functions better with the acid sensitive groups than the traditional methods using isobutene. Less hindered hydroxyl group of a diol can be protected and also is amenable to small scale work (avoiding the handing of gaseous isobutene). The t-butyl 2,2,2-trichloroacetimidate 36 is readily prepared by the addition of t-butanol to trichloroacetonitrile. Most of the experiments were carried out in presence of a mixture of dichloromethane and cyclohexane. Acetic anhydride in presence of boron trifluoride etherate has been utilized for the of acetylation of hydroxyl group.24
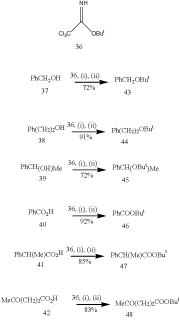
Figure 9 Conversion of alcohols and acids from t-butyl derivatives.
Reagents: 36, (i) BF3.Et2O, (ii) CH2Cl2, C6H12
Cyclization
The boron trifluoride etherate has played an important role in cyclization of many, carboxylic acids, allenes etc. The following few examples will illustrate the role of boron trifluoride etherate as cyclizing agent. The acid chloride 49 and alkene 50 were condensed to yield divinyl ketone25 51 which underwent Nazarov cyclization26,27 furnishing cyclic ketone 52 which was converted to the sesquiterpene trichodiene 53 (Scheme 10).
Several allenyl aryl ketones undergo cyclization with boron trifluoride etherate affording methylene benzocyclopentenone via a new 5-endo-mode cyclization.28 The ketones 54-56 afforded benzocyclopentenones 57-59 respectively (Scheme 11). Probably the transformation occurred as shown in the cyclization of allenyl aryl ketone 54 into 57. It can be observed that the presence of substituent groups in aromatic ring determines the yield of the cyclized product. Kos and Loewenthal28 reported the cyclization of the acid 60 with boron trifluoride etherate to the ketone 61 which was converted gibberone 62 (Scheme 12) in three steps:
In addition of the above mentioned reactions the following reactions also have been performed but not frequently employing boron trifluoride etherate.
(A) Thioketalization and ketalization
Thioketalization and ketalization reaction are very important reactions for organic synthesis. Some examples are given below. The acid 63 (Scheme 13) on esterification and thioketalization respectively afforded the compound 64 which on metal hydride reduction and acetylation respectively yielded the acetate 65. The conversion of the acetate into the alcohol 66 was accomplished in three steps:
The alcohol 66 was utilized for synthesis 29 of kaurenediol 67.
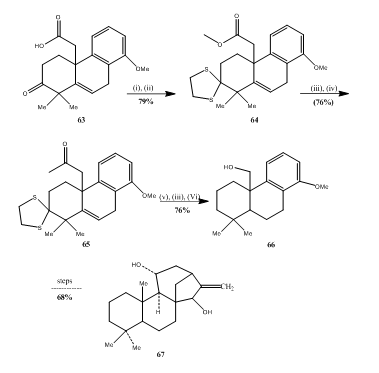
Figure 13 Synthesis of Kaurenediol 67
Reagents: (i) CH2N2, Et2O, (ii) (CH2SH)2, BF3OET2, (iii) LiAlH4, ET2O (iv) AC2O, Py, (V) Ra-Ni, vi) H2, 10% Pd/C.
Thioketalization reaction was also applied30,31 for the synthesis of non-aromatic abietane type quinone royleanone which shows the cytotoxic activity against Eagle’s KB strain of human carcinoma of the naso-pharynx. The synthetic route is described in (Scheme 14). The ketone 69, prepared, from the ketone 68, was subjected to thioketalization to obtain the compound 70. Desulphurization followed by catalytic hydrogenation yielded 71 whose conversion to royleanone 72 was accomplished in three steps:

Figure 14 Synthesis of non-aromatic abietane type quinone
Reagents: (i) (CH2SH)2, BF3.Et2O; (ii) Ra-Ni, boiling ethanol; (iii) 10% (Pd-C), MeCOOH; (iv) BBr3, CH2Cl2; (v) C6H6, O2; (vi) (MeCHCO2)2
Like thioketalization, boron trifluoride etherate has been used as catalyst for the reaction of ketones with oxirane to yield ketals.35 Thus the ketones (73-75) were converted to the ketals (76-78) respectively in high yield (Scheme 15). The use of epoxides for ketal formation offers the advantage of being extremely gentle, operating at room temperature and under protic conditions. Diethylene ortho carbonate converts ketones and aldehydes into their corresponding acetals in good yields at room temperature using slightly wet boron trifluoride etherate.36 It is particularly suitable for ortho-hydroxy aromatic aldehyde.
(B) Molecular rearrangements
Many organic compounds undergone transformation in presence of boron trifluoride etherate. The following examples illustrate the rearrangements originated by boron trifluoride etherate. The acetate 80 prepared from hydroxyl tetralin 79 underwent Fries rearrangement37 on heating at 125°C with boron trifluoride etherate in a microwave oven. The resulting tetrahydronaphthol38 81 (Scheme 16) was methylated to yield the compound 82 and finally converted to the already reported39 tetralone 83 (Scheme 16). Thus an alternative route38 was developed for the tetralone 83 which proved very useful for the synthesis39 of bioactive diterpene (+) triptoquinone A and its analog (+) triptin. Similarly, the Fries rearrangement with boron trifloride etherate was applied40 to the acetate, prepared from the naphthol 84. The compound 85 was treated with cerium ammonium nitrate (CAN) to obtain the quinone 86. The quinone was utilized for the synthesis of eleutherins40 87 and isoeleutherin 88 (Scheme 16).
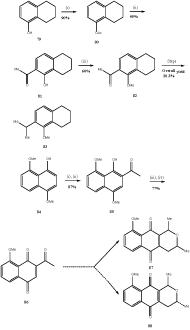
Figure 16 Synthesis of quinones from Naphthol
Reagents: (i) Ac2O, Py; (ii) BF3.Et2O; (iii) Me2SO4, Me2CO; (iv) CAN, MeCN
Matsumoto and collaborators41 reported a novel rearrangement of hydrophenanthhrene into hydroanthracene with boron trifluoride etherate. Thus the alcohol obtained by the metal hydride reduction of the enone 89 on treatment with boron trifluoride etherate suffered an interesting rearrangement yielding anthracene 90. This method for the synthesis of anthracene was applied to the synthesis of biologically active linear abietane diterpenes. Deslongchamps42 reported a very interesting rearrangement of diketone 91 to 8-acetoxy-4-twistanone 92 on treatment with a mixture of acetic acid, acetic anhydride and boron trifluoride at room temperature. The compound 92 is the first twistane derivative having a functional group at a bridge position (Scheme 17).
Srikrishna and collaborators43 reported one-step method for the conversion aryl ketones into the corresponding hydrocarbons by stirring with sodium cyanoborohydride in presence of two to three equivalents of boron trifluoride etherate. Thus the ketones (93-95) were converted into the corresponding hydrocarbons (96-98) respectively in high yield. These reactions were conducted at room temperature. High temperature was also used for the deoxygenation of some aryl ketones e.g. (99-101) into the hydrocarbons (102-104) respectively (Scheme 18).
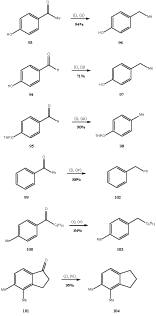
Figure 18 Conversion of the aryl ketones into the corresponding hydrocarbons
Reagents: (i) NaCNBH3, BF3.OEt2; (ii) RT, 12h; (iii) RT, 8h; (iv) 65ºC, 8h; (v) 65ºC, h; (vi) 65ºC, 4 h
A mixture of boron trifluoride etherate and acetic anhydride at 0o or below has been found to cleave different types of steroidal methyl ethers.44 Allylic and homoallylic ethers give the corresponding acetates in high yield. The completely saturated ethers give the acetate with retention of configuration as the main substituent product, but the epimeric acetate and elimination products are also obtained. A very interesting result was obtained when the demethylation experiment was tried with 4,8-dimethoxy-1-tetralone45 105 with boron trifluoride etherate and acetic anhydride. The tetralone underwent aromatization yielding the acetate 106 not the expected acetate107 (Scheme 19). On similar treatment the tetralone 108 afforded the acetate 109. These observations did not agree with the reported observation of Narayanan and Iyer on steroidal ethers.44
One of the most interesting applications of boron trifluoride etherate consists in the unprecedented alkylation of carboxylic acids in the absence of alcohols.46 Many aromatic and aliphatic carboxylic acids were readily alkylated in short time. It was also observed that ortho-substituted hydroxyl groups of carboxylic were not affected by alkylation but those of Meta-and para-substituted carboxylic acids were partially etherified. In addition alkylation reaction was carried out in presence of several functional groups such as halogens, amino and nitro groups. Some examples are given below (Scheme 20). Thus the acids (110-112) were alkylated to obtain the alkylated products (113-115) respectively.
This mini review focuses some of the applications of the boron trifluoride etherate in organic synthesis. The use of boron trifluoride etherate would continue owing to its operational simplicity and low cost. We believe the many of the above mentioned reactions could not have been accomplished without boron trifluoride etherate. Some of the products mentioned above have received use in natural product synthesis. It is necessary to indicate that many important applications of boron trifluoride etherate have been omitted in this review due to the limitation of space. We are sure that the information’s discussed would serve the need of organic chemists engaged in searching new applications of boron trifluoride etherate in organic synthesis.
The authors gratefully acknowledge the receipt of the reprint on alkylation from Dr. Wayiza Masamba, Department of Chemical and Physical Sciences, Walter Sislu University, Nelson Mandela Drive, South Africa and collaboration of Mr. José Gregorio, Bibleoteca IVIC, Caracas Venezuela for literature Po S. Poon also thanks CONICYT PIA/APOYO CCTE AFB170007 and CONICYT FONDECYT 1161068.
Authors declare that there is no conflict of interest.

©2019 Banerjee, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.