MOJ
eISSN: 2574-9722


Research Article Volume 8 Issue 4
1Master in Sciences, Biomedicine, Chemistry and Biotechnology, Researcher and Consultant at Goldlab Science and Technology, Brazil
2Biomedicine student at UPP – Pequeno Príncipe University, Microbiologist, Toxicologist and Technical Manager at Goldlab Ciência e Tecnologia Ltda, Brazil
Correspondence: Flavia Caroline Haluch, Biomedicine, student at UPP – Pequeno Príncipe University, Microbiologist, Toxicologist and Technical Manager at Goldlab Ciência e Tecnologia Ltda, Brazil
Received: October 07, 2023 | Published: October 25, 2023
Citation: Silvia MH, Flavia CH. Microplastics: toxicity and Bioacumulation at the first trophic levels of the food chain. MOJ Biol Med. 2023;8(4):159-164. DOI: 10.15406/mojbm.2023.08.00203
Microplastics are dispersed in nature in all planetary compartments, and can be found in the most remote places of the Ecosystem. They are currently indicated as markers of the Anthropocene, a geological era caused by human changes on the globe. Even though it is found in all environmental compartments, there are still gaps in scientific knowledge about its real impact on living cells and their dynamics, including in the human organism. Therefore, the objective of the study is to use an internationally standardized and standardized method in representative organisms of the ecosystem, thus using the acute and chronic ecotoxicity test with the microcrustacean Daphnia magna and for the algae Desmodesmus subspicatus, respectively, against polyethylene microspheres, a thermoplastic widely used as an exfoliation product and applied in various cosmetics and hygiene materials. They were tested in neonates according to the reference standards, but 30-day-old adult organisms were also exposed. The results obtained, for the crustaceans, show the Effective Concentration that causes effect in 50% of the organisms (EC50%), with 95% confidence of 0.07 g/L and a DSC – Observed effect concentration of 0.025 g/L for neonates. For the adult specimens, 0.025 g/L of EC50 and 0.0025 g/L of DSC were found, showing that the pups are 10x more resistant to plasticizers compared to the adults. For the algae test, the results showed a DSC of 0.05 g/L, inhibiting 20.12% of algae growth compared to the control. In the optical microscope of the crustaceans after exposure, it showed bioaccumulation of the polyethylene microsphere in the carapace and digestive tract of the bioindicator, showing that the impact of microplastics begins in the first trophic levels of the food chain.
Keywords: polyethylene, ecotoxicity, plasticizers, anthropocene, ecosystem
Plastics, microplastics and nanoplastics are classified as primary and secondary in origin,1,2 with the first originating and produced industrially, known as synthetic thermoplastics, and the second, originating decomposition of primary macroplastics or microplastics.3–5 Microplastics are produced in microscopic sizes, typically from 1.2 to 5 mm in diameter, used for numerous industrial purposes and replace other materials such as glass, wood and metals.3,6,7 The so-called secondary microplastics are formed by the fragmentation of primary plasticizers and their accumulation in aquatic ecosystems, occurring through the grouping of different synthetic polymeric materials.5,8
Due to extensive application in various chemical areas combined with inadequate disposal, microplastics and nanoplastics are being accumulated in the ecosystem as a whole, and especially in the oceans, aquatic organisms and sediments Wole et al. and 9,10 described that one of the inputs of these contaminants into water comes from washing synthetic fabrics, serving as an indicator of river pollution, linked to the impact of sanitary discharge. These particles reach rivers and oceans via runoff,11 traveling long distances until they reach the seas.3,5,6,12,13 It is impossible to ignore the effects of these components on aquatic life, as they accumulate continuously and gradually. The raw material for the production of plasticizers is derived from petroleum, which after refining processes results in the formation of monomers and polymeric resins called “pellets”, being a matrix for a wide variety of thermoplastics with different characteristics and uses.8 The plasticizers of great international prominence are Polyvinyl chloride (PVC), Low-density Polyethylene (LDPE), High-density Polyethylene (HDPE), Polypropylene (PP), Polyethylene terephthalate (PET) and Polystyrene (PS).10,13,14
In recent years, several authors have reported the presence of microplastics in different geographic locations, including in the most remote places on the planet, as well as in human consumption products, organs and tissues, human blood, animals and fetuses, generating growing concerns in the scientific community. 6,9,12–16 The first report of plasticizers in the marine environment was in 1970 and the term microplastics was created by Thompson and his collaborators in 2004.17,18 From 2005 to the present day, scientific studies have reported on the impact of microplastics on lower trophic organisms and their vast distribution in all compartments of the planet, which include the gaseous atmospheric layer, ice, soil, water, sediment, plants, organisms, that is, a global distribution.6,9,14–16 Since the term “microplastics” was created in 2004 until today, there have been 19 years of planetary exposure of a synthetic product, that is, produced anthropically. We know that geological eras are time intervals caused by billions of years of geological changes, contributing to a specific natural characteristic. Silva et al.8 and Coppok et al.19 described that microplastics are the great indicators of the Anthropocene, that is, the era of impacts caused by man. Therefore, the objective of the study is to use standardized methodologies, standardized and published by regulatory organizations in the field of Ecotoxicology, in order to observe the toxicity of blue polyethylene microspheres against the bioindicator Daphnia magna and Desmodesmus subspicatus with the aim of contributing to discussions about of the global impact and trends of the Anthropocene.
Ecotoxicology
Ecotoxicology is a scientific tool that can be understood as the study of the interaction of living beings with each other and the effects caused by some substance or change in the environment, which impacts biological processes and causes deleterious effects at some trophic level of the food chain. The objective of ecotoxicology is to understand the effects of substances or factors on living beings or natural communities. Therefore, representatives of the ecosystem are needed to simulate, under laboratory conditions, events that can be extrapolated to the natural environment.20
An excellent bioindicator for ecotoxicity tests is the microcrustaceanDaphnia magna (Figure 1), represents zooplankton (approximately 6 mm) and is classified as Arthropoda, subphylum Crustacea, class Branchiopoda, order Displostraca, suborder Cladocera, having an ideal life cycle for maintenance in laboratories and reproduces genetically by bipartition, that is, forming generations identical to the original matrix, and this avoids genetic variations and consequently, universal data repetition.20–23 Another bioindicator of great international relevance is green algae,Chlorophyta, Chlorophyceae, represented by Desmodesmus subspicatus (Figure 2), are planktonic organisms (with approximately 20µm)and represent the primary producers in aquatic ecosystems, serving as food for zooplankton, in addition to maintaining the balance of the biogeochemical cycles of carbon, nitrogen, oxygen and phosphorus, in addition to being the basis of global atmospheric oxygen due to photosynthesis.24,25
Microplastic
The microplastics tested areblue polyethylene microspheres, CAS 8002-74-2 lot VA076, with an average of 2 mm in diameter Figure 3 and were purchased commercially from suppliers of products to be applied as exfoliants for skin tissue, body cream, toothpaste and soaps.
Bioassay
Acute ecotoxicity test with Daphnia magna
Ecotoxicity tests were carried out following the conditions of Standard ABNT NBR 12713–Aquatic ecotoxicology–Acute toxicity, test method with Daphina spp (Crustacea, Cladocera), similar to ISO 6341. Daphnia magna was cultivated in a water solution composed of basic medium called M4 which contains essential salts characteristic of natural water. The method is based on exposing Daphnia magna neonates to various dilutions for a period of 48 hours without lighting. After this time interval, swimming inability is observed in relation to the white.22,23
Each concentration was tested in duplicate and each beaker had a volume of 100 mL with 20 organisms placed. The beakers were isolated with plastic film and protected from light using a covered with aluminum foil and left in an air-conditioned room with a controlled temperature of 18 to 22ºC.23 The test solutions were based on exposures containing polyethylene microspheres, weighed on a high-precision analytical balance, at concentrations of 0.001; 0.005; 0.01; 0.025; 0.05; 0.07; 0.1; 0.25; 0.5; 0.7 and 1 g of material for 100 mL of M4 solution. Sensitivity tests were carried out to assess the quality of the specimens, using potassium chromate as a reference substance, to plot the control chart. The results are expressed in Effective Concentration that causes an effect on 50% of the organisms (EC50) represents the nominal concentration of the sample that causes an acute effect on 50% of the organisms during the exposure time through swimming inability. The statistical method for calculating EC50 was the TSK - Trimmed Spearman-Karber program, which is a method that aligns the calculations to 95% confidence.21
Chronic ecotoxicity test with desmodesmus subspicatus
The principle of toxicity testing with algae consists of evaluating the inhibition of algal growth, during exposure of the test sample with nutrient medium, to different sample solutions, for a period of 96 hours, in recipients that facilitate light penetration and properly covered with parafilm paper, preventing evaporation and placed randomly on a shaker at 100 rpm. Dilutions are made in triplicate and the test is maintained between 23 and 27º C with continuous luminosity above 4500 lux. The results are based on the count performed by microscopy on a Sedgewick rafter slide and to determine the toxicity factor (TF) and the percentage of inhibition of algal biomass growth by 50% (CIP50), it is calculated for each concentration by comparing the average growth rate between replicates of the test solutions with the average obtained from the control.25 The test solutions were based on exposures containing polyethylene microspheres, weighed on a high-precision analytical balance, at concentrations of 0.001; 0.005; 0.01; 0.025; 0.05; 0.07; 0.1; 0.25; 0.5; 0.7 and 1 g for 100 mL of solution.
Chromatographic test
The volatile analysis followed thegas chromatography (GC) technique using an FID detector (flame ionization detector), DB624/DB5 column, nitrogen gas, hydrogen and synthetic air of purity 5. The analytical technique was performed by Headspace and thesample volume used in the chromatographic test was 10 mL and transferred with a micropipette, calibrated RBC - Brazilian calibration network, to the bottle with a screw cap - Part Number: 5188-2759 - Agilent Technologies. After adding the samples, the vial was closed and kept in a 90ºC oven for 1 hour EPA 3890. The test aims to investigate whether the microspheres release any volatile components in the white exposure solutions and the highest concentration vat of the Daphnia magna test.
Quality control of ecotoxicological tests
Blank tests were carried out and the results are in accordance with standardized standards, with no specimen deaths occurring. The sensitivity tests were plotted on a control chart (Figure 4 & 5), and the results were ideal, without trends, bringing reliability to all results obtained. The results of the toxicity tests for blue polyethylene microspheres are described in Table 1 for Daphnia magna and Table 2 for Desmodesmus subspicatus.
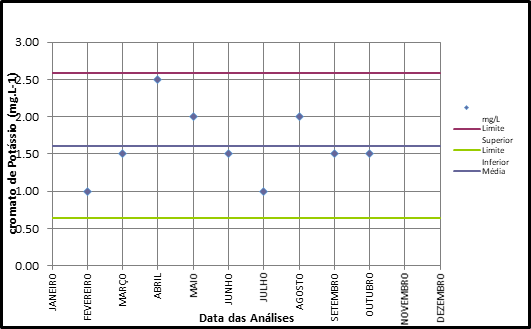
Figure 5 Desmodesmus subspicatus sensitivity control chart with potassium chromate.
Source: Authors.
|
Neonates |
Adults |
|||||
|
Dilutions |
||||||
|
20 organisms in 48 hours |
Test 1 |
Test 2 |
Average |
Test 1 |
Test 2 |
Average |
|
|
Number of deaths |
Number of deaths |
Number of deaths |
Number of deaths |
Number of deaths |
Number of deaths |
|
White |
0 |
0 |
0 |
0 |
0 |
0 |
|
0.001 g/100 mL |
0 |
0 |
0 |
0 |
0 |
0 |
|
0.005 g/100 mL |
0 |
0 |
0 |
0 |
0 |
0 |
|
0.01 g/100 mL |
0 |
0 |
0 |
0 |
0 |
0 |
|
0.025 g/100 mL |
0 |
0 |
0 |
two |
two |
two |
|
0.05 g/100 mL |
0 |
0 |
0 |
3 |
4 |
3 |
|
0.07 g/100 mL |
0 |
0 |
0 |
5 |
6 |
6 |
|
0.1 g/100 mL |
1 |
1 |
1 |
8 |
8 |
8 |
|
0.25 g/100 mL |
3 |
3 |
two |
10 |
10 |
10 |
|
0.5 g/100 mL |
5 |
6 |
6 |
13 |
14 |
14 |
|
0.7 g/100 mL |
10 |
10 |
10 |
20 |
20 |
20 |
|
1 g/100 mL |
19 |
18 |
20 |
20 |
20 |
20 |
Table 1 Results of acute ecotoxicity tests with microcrustaceans
|
Tests in 96 hours |
A |
B |
W |
Average |
% Cip inhibition |
|
cell/mL |
cell/mL |
cell/mL |
cell/mL |
||
|
White |
15,200 |
16,800 |
15,700 |
15.9 |
- |
|
0.001 g/100 mL |
15,850 |
15,600 |
14,900 |
15,450 |
2.83 |
|
0.005 g/100 mL |
15,720 |
14,850 |
14,520 |
15,030 |
5.47 |
|
0.01 g/100 mL |
15,620 |
14,500 |
15,100 |
15,073 |
5.2 |
|
0.025 g/100 mL |
15,200 |
15,100 |
14,800 |
15,033 |
5.45 |
|
0.05 g/100 mL |
14,780 |
14,900 |
15,200 |
14,910 |
6.22 |
|
0.07 g/100 mL |
14,520 |
14,750 |
14,500 |
14,590 |
8.23 |
|
0.1 g/100 mL |
13,200 |
13,100 |
13,800 |
13,366 |
15.94 |
|
0.25 g/100 mL |
13,100 |
12,800 |
12,900 |
12,933 |
18.66 |
|
0.5 g/100 mL |
12,800 |
12,550 |
12,750 |
12,700 |
20.12 |
|
0.7 g/100 mL |
11,800 |
11,200 |
11,500 |
11,500 |
27.67 |
|
1 g/100 mL |
10,800 |
10,750 |
10,600 |
10,716 |
32.6 |
Table 2 Results of chronic ecotoxicity tests with algae
Given the results, since they aimed at swimming incapacity, following the ISO standard, normalized and standardized in acute tests with microcrustaceans, we can show that the Concentration of no observed effect - CENO, for neonates, are below 0.07 g /100 mL (0.007 g/L) up to 0.001 g/100 mL and the Concentration of observed effect – CEO, with at least 10% deleterious effect, is in the order of 0.25 g/100 mL (0.025 g/L) . For 30-day-old adults, the CENO is below 0.01 g/100 mL (0.001 g/L) up to 0.001 g/100 mL and the CEO at 0.025 g/100mL (0.0025 g/L). Another event observed in the tests carried out, mDespite the chemical characteristic of plasticizers, which have a low density and consequently float in the liquid medium, it was observed that after the death of the organisms, they decanted, which explains why microplastics are found in river and ocean sediments. For EC50 which is the concentrationEffective that causes an effect on 50% of the organisms tested, we have 0.7 g/100 mL (0.07 g/L) for neonates and 0.25 g/100 mL (0.025 g/L) for exposed adults. In this way, we can observe that adults are on average 10x more sensitive to microplastics compared to newborns, which demonstrates a regulatory gap, when aiming to expose only young people in toxicity tests with microcrustaceans in a standardized way.
Zhang et al.12 used RNA genetic sequencing to evaluate gene expression in 21 days of chronic exposure to polystyrene nanoplastics in Daphnia pulex and the results showed 244 altered genes, in addition to delayed growth, there was a change in reproductive capacity and a change in the sex ratio of newborns. The same scientist and his collaborators, Zhang et al.6 describe that crustaceans are extremely important in the transmission of energy and that microplastics alter physiological and behavioral functions. When microplastics coexist with other contaminants, small amounts cause a synergistic impact, posing an ecological threat to these aquatic organisms.
Besseling et al.14 studied polystyrene nanoplastic with Daphnia magna. For the same indicator, the effects of altering the body size of Daphnia magna were found at 103 mg/L and from 30 mg/L of polystyrene nanoplastic, newborns were poorly formed and reproduction decreased by 68% of offspring. In addition to evaluating swimming inability, the exposed specimens were observed under optical microscopy to analyze the effects of bioaccumulation as shown in Figure 6. From the images we can see that the neonate fed on microplastic, as internally in its digestive tube (blackened part) it contains microplastics (Figure 6B), which can be compared with (Figure 6A), which is the neonate before exposure, that is, in natura , containing only planktonic green algae, which is its regular diet. Figure 6C, 6D & 6E of adults, also showed bioaccumulation between the internal membranes and within the siliceous carapace of the specimens after exposure. In (Figure 6D), it is even possible to observe the formation of eggs close to the internally adhered microplastic sphere and finally, Figure 6E, shows the rupture of membranes due to the excess bioaccumulation of the blue spheres and consequently, a collapse of the ecdyses, which these are the changes in the chitinous exoskeleton, common in arthropods.
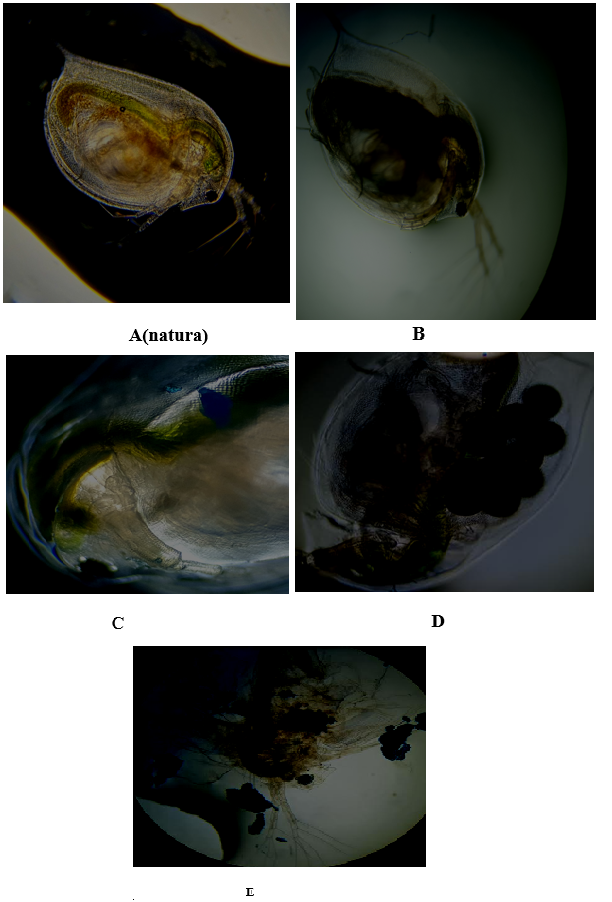
Figure 6
6A: Daphnia magna neonate in natura.
6B: Daphnia magna neonate after 48 hours of exposure, containing microplastics in its digestive tube.
6C &6D: Adult Daphnia magna, 30 days old, with 24-hour exposure, with evident bioaccumulation in the carapace. 6E. Bursting of the carapace due to excess microplastics.
Therefore, there is no way to dispute the bioaccumulation of microplastics at the trophic level of primary zooplankton consumers and, consequently, transfer to other levels of the food chain. In the case of primary producers represented by the green alga Desmodesmus subspicatus, the analytical results are described in the table. From the tests carried out, we observed that the CEO is 0.5g/100 mL (0.05g/L), presenting an inhibition of 20.12%, which, according to the standardized norm, is necessary for at least 20% to occur in relation to the control, for the assay to be expressive and validated, with this concentration being the toxicity factor – TF. However, up to a concentration of 1g/100 mL (0.1g/L) CIP50 was not observed for the organism studied under these laboratory conditions.
The most concrete fact observed in laboratory tests, is that the microplastic impaired the penetration of light into the tubes and consequently reduced photosynthesis due to the chlorophyll present in the green algae and, finally, reduced its growth in relation to white algae. As the test organism is small in size, the microplastic may not have interfered with its metabolism directly, just a physical event of light blocking. However, this is extremely serious since in Nature, the sun's rays are unable to penetrate the liquid layer and consequently reduce the production of atmospheric oxygen, a precious asset for maintaining life on the planet. But scientists Besseling et al.14 studied polystyrene nanoplastic with the bioindicator Scenedesmus obliquus and observed that at a concentration of 0.22 mg/L polystyrene reduced chlorophyll growth in relation to the control, therefore, minute plasticizers induce harmful effects on phytoplankton at the cellular level. To complete the laboratory tests and seek to understand the impacts on microplastics in aquatic compartments, the solutions tested after 48 hours of exposure to Daphnia magna, the blank and the last solution of 1g/100 mL were tested for volatiles and the chromatograms can be seen in (Figure 7A & 7B).
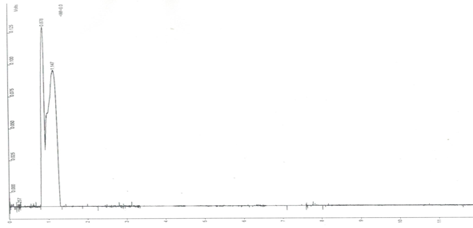
Figure 7A Chromatogram of the test blank with microplastic-free Daphnia magna (1: methane; 2: humic acid).
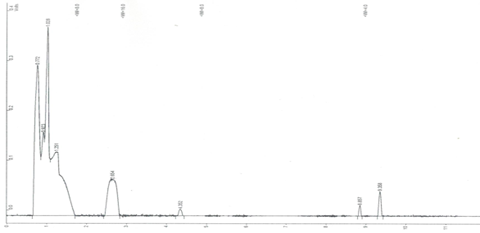
Figure 7B Chromatogram of the 1g/100 mL test solution with Daphnia magna. (1: methane; 2: formaldehyde; 3: humic acid, 4: Ethene; 5: Ethylene; 6 and 7: phenolic compounds of the indigo dye).
Based on the results obtained from the chromatographic runs, microplastics, in addition to being ingested as food by the organisms tested, also release substances into the liquid medium that provide synergistic effects, that is, they stimulate more harmful effects combined with all their impacts on the ecosystem. In addition to releasing plasticizer monomers, the dyes used release phenolic compounds that have the benzene ring, that is, the phenol and benzene compound can be released and both have one of the lowest maximum limits allowed in surface and groundwater, of 2 and 5µg/L, respectively, being the reference value adopted internationally.26 The impact of microplastics on the planet is being reported as a new geological era by several authors. Santos et al.27–29 found new rock formations, called plastiglomerates, similar to sedimentary rocks and plastistones, similar to igneous rocks, found on the island of Trindade in Espirito Santo, Brazil, demonstrating the size of the environmental impact of these components on the ecosystem. Therefore, it is impossible to deny the harmful effects of this industrial component, that is, synthetic and produced by human activities, spread across the globe and beyond, through satellites and interplanetary waste.
Based on the data obtained, we can state that the dynamics of microplastics in the environment causes serious deleterious effects on one of the main trophic levels of the food chain, which are crustaceans. It is known that in all planetary compartments we can find these components that are currently indicated as a model of a new geological era caused by man, called the Anthropocene. Waste management is extremely important, with its reuse and recycling, with environmental policies focused on sustainability indicators, selective collection and environmental education.
We thank Goldlab Science & Technology and Huhtamaki Nederland BV and Huhtamaki Fiber Packaging Brazil.
The authors declare that there is no conflict of interest.
The work was funded by the Fiber Packaging Brazil and thanks for being a company committed to environmental issues and science for the world.

©2023 Silvia, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.