MOJ
eISSN: 2574-9722


Research Article Volume 5 Issue 1
1Independent researcher, retired from the Biology of Organisms Department, University of Brussels, Belgium
2Independent researcher, retired from the Natural and Agricultural Environmental Studies Department (DEMNA) of the Walloon Region, Belgium
Correspondence: Marie-Claire Cammaerts, independent researcher, 27, Square du Castel Fleuri, 1170, Brussels, Belgium, Tel 3226 7349 69
Received: October 09, 2020 | Published: October 15, 2020
Citation: Cammaerts MC, Cammaerts R. Ethological and physiological side effects of oxybutynin studied on ants as models. MOJ Biol Med. 2020;5(1):4?16. DOI: 10.15406/mojbm.2020.05.00116
Patients suffering from urinary incontinence are still nowadays mostly treated with oxybutynin. Using ants as models, we found that this drug decreased their food consumption, orientation ability, tactile perception, cognition and memory, induced restlessness and stress, impacted their social relationships and leaded to dependence. No adaptation occurred to these side effects. The action of oxybutynin quickly vanished in 10 hours. Most of these side effects corresponded to those observed in humans (on whom effects on food consumption, activity, cognition and anxiousness can be observed), and some others were observed in ants (impact on social relationships, dependence on the drug and absence of adaptation to its side effects). Ants appear thus to be valuable models for revealing side effects of a drug. On the basis of our results and of those reported in the literature, it can be concluded that patients treated with oxybutynin should be carefully monitored as for their risk of developing adverse effects, even unexpected ones. Novel, safer medicines, presenting a better balance between efficacy and safety should be researched.
Keywords: dependence, food consumption, memory, restlessness, urinary incontinence
ang.deg., angular degrees; ang.deg./cm, angular degrees per cm; mm/s, millimeter per second; χ², chi-square; vs, versus; n°, number; cm, centimeter; mm, millimeter; ml, milliliter; mg, milligram; s, second; min, minute; h, hour; t, time; %, percentage
A frequent disabling health problem from which some children and elderly persons suffer is urinary incontinence due to overactive bladder (OAB) functioning. This syndrome is due to unconscious and involuntary contraction of the detrusor smooth muscle when the bladder fills. Since the detrusor muscle is innerved by the parasympathetic nervous system, the OAB syndrome is essentially treated with anticholinergic drugs: these drugs block the neurotransmission of acetylcholine to the muscarinic detrusor muscle receptors. The problem is that acetylcholine acts also as a neurotransmitter in many other organs. Thus, although the muscarinic receptor subtype M3 is predominantly responsible for the detrusor contraction, antimuscarinic drugs act also on all the five muscarinic receptor subtypes (M1–5) present throughout the body, what may lead to unwanted effects. All these receptors are expressed in the brain, including in the hippocampus. M1 receptors appear to be dominant and primarily involved in cognitive functioning (e.g. attention, learning and memory), are also present in glands and in sympathetic ganglia and are facilitatory receptors on the cholinergic nerve endings of the detrusor. M2 receptors also play a role in cognition and memory processes and are furthermore present in the heart and smooth muscles. M3 receptors are present in brain, smooth muscles, salivary glands and eyes. M4 receptors are present in the basal forebrain and are inhibitory receptors in the endings of the nerve innervating the detrusor muscle. M5 receptors are present in projection neurons of the brain substancia nigra, and also in the ciliary muscle.1–4 The M3 subtype accounts for one-third of the muscarinic receptors in the bladder where their stimulation by acetylcholine is predominantly responsible for the detrusor contraction. Two-thirds of the muscarinic receptors in the bladder are of the M2 subtype, their stimulation reversing the sympathetically β-adrenoreceptor mediated detrusor relaxation.5 Blockade of M2 and M3 receptors by antimuscarinic drugs reduces leakage episodes and the number of daily voids and thus enhances quality of life. However, the blockade of the M3 receptors results also in dry mouth, constipation, blurred vision, and drowsiness.1 The available anticholinergic drugs lack selectivity for the detrusor receptors and may thus have adverse effects on many organs the innervations of which depends on acetylcholine as neurotransmitter.6 Forty of these adverse events resulting from antimuscarinic OAB treatment were recorded.7 However, each antimuscarinic drug has its own differential affinity for the M3 receptor compared with that for the other M receptors, what can be used in some degree to avoid undesirable adverse effects (see the Discussion section).
Overactive bladder is still mostly treated with oxybutynin chlorhydrate, an anticholinergic tertiary amine (Figure 1) with antispasmodic effects on the detrusor muscle. This drug was among the first anticholinergic medications used to treat OAB, being delivered on the American market since 1975 under an immediate release (IR) formulation8 and still remained, in 2017, the 100th most commonly prescribed medication in the United States (Wikipedia). A review of randomized controlled trials on the efficiency of anticholinergics compared with placebo treatment shows that oxybutynin significantly reduces urinary incontinence, but with the main awkward effects of increasing dry mouth, constipation and eye blurring.9 An experimental work on mice as models showed that, as in rats and humans, oxybutynin efficiently acts on the efferent pathway of the urinary reflex.10 Although being efficacious as a drug, oxybutynin has side effects so bothersome that some patients prefer to discontinue treatment and to endure the drawbacks of overactive bladder.8 Acomplete list of oxybutynin unwanted effects has been produced by the Drugs.com website,11 those requiring the most attention being eye pain, skin rash, dizziness and irregular heartbeat. Moreover, among the most frequently reported adverse reactions are dry mouth, skin impairments, cardiac arrhythmia and tachycardia, gastrointestinal discomfort, urinary retention, dizziness, somnolence, tiredness, insomnia, abnormal vision and respiratory discomfort. The instructions for use joined to oxybutynin packages also mention periodontal impairment due to long lasting use, urinary infection, vision impairment, constipation, dizziness, sleep troubles and modification of the effects of other medicines because oxybutynin decreases defecation. A meta-analysis of literature reveals that oxybutynin is more lipophilic, has a lower degree of ionization and a relatively smaller molecular size and is thus more prone to cross the blood-brain barrier (BBB) than the other anticholinergic drugs (more prone than tolterodine, the less prone being fesoterodine and trospium chloride). Consequently, oxybutynin is expected to induce the more adverse effects on the central nervous system (CNS) such as cognition impairment, dizziness and somnolence.12,13 To warn for adverse CNS effects, the product labels for oral oxybutynin were modified in the United States in 2008.4 In Europa, the more easily available and still considered as the most efficient antimuscarinic drug remains oxybutynin. This is why, in the same way that we studied the effects of many drugs and products consumed by humans by using ants as a biological model (the effects of 38 products have already been examined [e.g.14–19]), we intended to use again an ant species as a model for studying the potential physiological and ethological side effects of oxybutynin, including its effects on memory, social relationships and sensory perception. We affirm having made our experimental work before looking for medical information on oxybutynin, i.e. we made our work being unaware of its possible adverse effects. Here below, we explain why using ants as models, which species was used, what we know about it, and which traits we aimed to examine.

Figure 1 Chemical structure of oxybutynin (its S-enantiomer is here figured), making a sugar solution of this medicine, drunk by two ants. Oxybutynin is an anticholinergic drug used to prevent urinaryincontinence in old persons and infants. A solution of this drug in sugar water corresponding to its amount daily consumed by humans was given to the ants (details in the text) instead of their usual sugar water.
Why using ants as models
Nearly all the biological processes are identical in every animal species including humans (e.g. genetics, embryology, molecular biology, anabolism and catabolism, nervous cells functioning …). Invertebrates and vertebrates are thus used as models for studying a lot of biological traits.20,21 Invertebrates are more and more used because they are small and easily maintained in a laboratory, have a short life cycle and a rather simple anatomy.22 Some species are widely used as models: for example, the flatworm Dendrocelium lacteum, the nematode worm Caenorhabdotes elegans, the mollusk Aplysia californica, the beetle Tribolim castaneum, the fruit fly Drosophila melanogaster, and the honeybee Apis mellifera. Insects and among them social hymenoptera offer scientific and easiness advantages and are thus largely used.23 Ants can thus be used as models, essentially because they possess many sophisticated biological traits. These eu-social insects among other particularities navigate using learned cues, recruit nestmates, differently mark parts of their territory, take care of their brood, build a complex nest, clean it and manage cemeteries at the frontiers of their territory.24 We use ants as models since a long time and have always found ethological features, physiological events, adverse effects of drugs and situations, general biological notions valuable for any animal species humans included. In the present work, we used once more the species Myrmica sabuleti Meinert, 1861.
What do we know about cognition in the used ant species
The workers of M. sabuleti can adapt their navigation as well as the choice of their nest site to their visual and olfactory perception, can adequately recruit nestmates, can recognize themselves in a mirror, at their emergence become imprinted to the appearance of the front head of their congeners, during their first year of life learn several behaviors in the presence of older congeners,25–27 natively possess a number line, acquire the notion of zero through experiences, and can acquire numerical symbolisms.28–30 Similarly to evolved species and humans, their perception and responses are influenced by the distance and the size effects, and the physiological law of Weber can be applied to them.31,32
The traits we examined
Using M. sabuleti as a model, we here examined the impact of oxybutynin on the workers’ food consumption, general activity, locomotion, orientation ability, audacity, tactile (pain) perception, social relationships, cognition, stress, learning ability and memory, as well as the adaptation to the side effects of the drug, the dependence on its consumption, and the decrease of its effect after its consumption was stopped. Our experimental protocols were identical to those previously used [e.g.33–35]. Therefore, we here only briefly explain them, being however unable to avoid inevitable plagiarism. Let us recall that we performed our entire experimental work ignoring the up to now known side effects of oxybutynin, having made our experiments being blind to this knowledge.
Collection and maintenance of ants
The experiments were performed on three colonies of M. sabuleti collected in September 2019 at Olloy/Viroin (Ardenne, Belgium) in an abandoned quarry. These colonies contained about 500 workers, a queen and brood, and were living in grass and under stones. Two colonies (A and B) were used to make the experimental work and a third colony C was used for performing the control of the conditioning experiment. These colonies were maintained in the laboratory in one to three glass tubes half filled with water, a cotton plug separating the ants from the water. These nest tubes were deposited in a tray (34cm x 23cm x 4cm) which served as a foraging area, in which a 30% aqueous solution of sugar was permanently provided in a tube plugged with cotton, as well as pieces of Tenebrio molitor larvae (Linnaeus, 1758) deposited three times per week. While working on ants, the lighting of the laboratory equaled about 330 lux. The ambient temperature continuously equaled ca 20°C, the humidity ca 80%, and the electromagnetism 2 µWm2, three conditions suiting for the used species. The ants are here often named workers or nestmates as commonly do researchers on social insects.
Solution of oxybutynin given to the ants
A package of oxybutynin chlorhydrate IR (immediate release formulation) produced by EUROGENERICS s.a. (1020 Brussels) was furnished by the pharmacist Wera (Brussels, Belgium). Humans treated with this drug are advised to consume each day 2 to 3 tablets containing 5mg of oxybutynin. Thus, in agreement with the instruction for use joined to the drug package, they generally consume 2 tablets of oxybutynin (= a total of 10mg of the drug) each day, absorbing at the same time about one liter of water. Insects, and thus ants absorb about ten less water than mammals according to their physiology and anatomy. Consequently, to maintain ants under an oxybutynin diet similar to that of humans, they must be provided with a solution of 10mg oxybutynin in 100ml of water, or in the present case, in the sugar water delivered in the ants’ usual sugar water cotton plugged tubes (Figure 1). The cotton plug of these tubes was refreshed every 2-3 days, and the entire solution was renewed every 7 days. It was checked each day if ants drunk the provided solutions, what they effectively did. We firstly made all the control experiments on colonies A and B and the control conditioning experiment on colony C, while these colonies were maintained under normal diet. After that, test experiments were performed on colonies A and B provided with the solution of oxybutynin, starting one day after the ants’ drug consumption.
Food consumption, general activity
For normal diet as well as for a diet with oxybutynin, six times per day during six days (i.e. four times during the day and twice during the night, each day at the same times o’clock), the ants of colonies A and B present on the meat food, located at the entrance of the sugar water tube, as well as being active at any place of their territory (foraging area, nest entrance, inside of the nest) were counted. For each kind of diet and each kind of trait (eating meat, drinking, being active), the mean of the 6 times X 2 colonies = 12 counts was established (Table 1, the six first lines). The means of the six successive daily means were also calculated (Table 1, last line). For each kind of trait, the six daily means obtained for the oxybutynin diet were compared to the six daily means obtained for the normal diet using the non-parametric test of Wilcoxon,36 the level of significance being set at P=0.05.
|
Days |
Normal diet |
Diet with oxybutynin |
||||
|
meat |
sugar water |
activity |
meat |
sugar water |
activity |
|
|
1 |
1 |
1 |
9.5 |
1 |
1 |
9 |
|
2 |
1.5 |
2 |
9.5 |
0.5 |
1.25 |
9.5 |
|
3 |
1.25 |
2 |
8 |
0.5 |
0.67 |
11.75 |
|
4 |
1.5 |
2.75 |
9 |
0.75 |
0.5 |
12.75 |
|
5 |
1.5 |
1.5 |
10.5 |
0.5 |
0.5 |
12.33 |
|
6 |
1.5 |
2 |
11.5 |
0.25 |
0.75 |
13 |
|
mean |
1.38 |
1.88 |
9.63 |
0.58 |
0.78 |
11.39 |
Table 1 Impact of oxybutynin on the ants’ food consumption and general activity
The table gives the means of counted ants. The drug decreased the ants’ meat and sugar water consumption but increased their general activity. The ants were obviously more excited and nervous than usually. Details and statistics are given in the text
Linear and angular speeds, orientation
These three traits were quantified on ants moving in their foraging area, the speeds without stimulating the ants, the orientation while stimulating them with a nestmate tied to a piece of paper (Figure 2A). A tied nestmate emits its attractive mandibular glands alarm pheromone. For assessing the ants’ speeds during one experiment and the ants’ orientation during another experiment, the trajectory of 40 foragers were recorded and analyzed using appropriate software37 according to the following definitions. The linear speed (in mm/s) is the length of a trajectory divided by the time spent to travel it; the angular speed (in angular degrees per cm, ang.deg./cm) is the sum of the angles made by successive adjacent segments, divided by the length of the trajectory; the orientation (in ang. deg.) towards a location is the sum of successive angles made by the direction to the location and the direction of the trajectory, divided by the number of angles measured. When an animal tends to orient itself towards the location, its obtained value of orientation is lower than 90°; when an animal tends to avoid the location, its obtained value of orientation is larger than 90°. For each variable (linear speed, angular speed, and orientation), the median and quartiles of the distribution of 40 obtained values were established (Table 2, lines 1–3). The distributions obtained for ants under oxybutynin were compared to the corresponding distributions obtained for ants under normal diet by using the non-parametric χ² test.36
|
Traits |
Normal diet |
Diet with oxybutynin |
|
Linear speed (mm/s) |
12.2 (10.6–13.3) |
9.6 (8.2–11.0) |
|
Angular speed (ang. deg./cm) |
137 (119–146) |
150 (133–176) |
|
Orientation (ang.deg.) |
30.1 (22.7–47.4) |
60.4 (46.3–86.1) |
|
Audacity (n°) |
2.85 [2–4] |
3.50 [ 2 - 5] |
|
Tactile (pain) perception: |
||
|
linear speed (mm/s) |
5.6 (4.6–6.2) |
8.6 (7.7–10.2) |
|
angular speed (ang. deg./cm) |
227 (202–276) |
168 (136–193) |
Table 2 Impact of oxybutynin on five ants’ physiological and ethological traits
Oxybutynin increased the ants’ angular speed (and consequently reduced their linear speed), decreased their orientation ability and their tactile (pain) perception. It increased their audacity. The effects of oxybutynin on the ants’ locomotion and audacity are in agreement with that on their general activity (Table 1). Details on the methods and statistics are given in the text. mm/s: millimeter per second; ang.deg./cm: angular degree per centimeter; ang.deg.: angular degree; n°: number
Audacity
A cylindrical tower (height = 4 cm; diameter = 1.5 cm) tied to a squared platform (9 cm2), both in white Steinbach ® paper, was deposited in the ants’ foraging area.33-35 The ants present at any place on this apparatus were counted 10 times over 10 min (Figure 2B). The numbers obtained for the two colonies were pooled, and the mean and extremes of the recorded numbers were established (Table 2, line 4). To analyze the results, the numbers of ants of the two colonies counted during every two successive minutes were added, and the five sums obtained for ants under oxybutynin diet were compared to the five sums obtained for ants under normal diet by using the non-parametric test of Wilcoxon.36
Tactile (pain) perception
Under normal maintenance, the ants moving on a rough substrate perceive its uncomfortable character and walk then with difficulty, slowly, sinuously, often touching the substrate with their antennae (Figure 2C). An ant poorly perceiving the uncomfortable character of a rough substrate walks there with less difficulty, more quickly and less sinuously than ants under normal maintenance. Therefore, to assess the ants’ tactile perception, a folded piece (3cm x 2 +7+2=11 cm) of emery paper n° 280 paper was tied to the bottom and the borders of a tray (15cm x 7cm x 4.5cm) which was so divided into a first 3 cm long zone, a second 3 cm long zone covered with the emery paper, and a last 9 cm long zone. For making an experiment on one colony, 12 ants of that colony were deposited in the first zone of the apparatus, and their trajectories on the emery paper were recorded. Two colonies being used, 24 trajectories were thus recorded, and the linear and angular speeds of ants walking on the rough substrate could be quantified as usual (see subsection relative to linear and angular speeds). For each variable and each kind of diet, the median and quartiles of the distributions of the 24 obtained values were established (Table 2, lines 5, 6). The distributions obtained for ants consuming oxybutynin were compared to those obtained for ants under normal diet by using the non-parametric χ² test.36
Brood caring
A few larvae of each colony were removed from the inside of their nest and deposited outside, at one to three centimeters from the entrance. For each colony, five of these larvae were observed during five minutes (Figure 2D), and those among these 5 larvae not re-entered in the nest after 30 seconds, 1, 2, 3, 4 and 5 minutes were counted. The numbers obtained for the two colonies were added (Table 3, line 1). The six sums obtained for ants consuming oxybutynin were compared to the six sums obtained for ants under under normal diet using the non-parametric test of Wilcoxon.36
|
Traits |
Normal diet |
Diet with oxybutynin |
|
Brood caring: n° of not re-entered |
30s 1 2 3 4 5 |
30s 1 2 3 4 5 |
|
larvae over time |
8 6 4 2 2 0 |
10 10 10 10 8 8 |
|
|
|
|
|
Relation with nestmates: n° of |
0 1 2 3 4 ‘a’ |
0 1 2 3 4 ‘a’ |
|
aggressiveness levels; variable ‘a’ |
79 41 15 0 0 0.05 |
24 39 42 0 0 0.67 |
|
Cognition: n° of ants over time in front and beyond the difficult path |
2 4 6 8 10 12min |
2 4 6 8 10 12min |
|
|
in front 17 16 11 9 8 6 |
in front 23 19 16 14 17 13 |
|
|
beyond 0 0 3 4 6 6 |
beyond 0 0 0 1 1 2 |
|
Escaping ability: n° of ants among |
2 4 6 8 10 12min |
2 4 6 8 10 12min |
|
12 escaped over time |
2 4 4 6 7 9 |
0 0 1 2 2 4 |
Table 3 Impact of oxybutynin on four ants’ physiological and ethological traits
Oxybutynin affected the ants’ social relationships (their brood caring and behavior towards nestmates, lines 1, 2), as well as their cognitive abilities (cognition and escaping behavior, lines 3, 4). The latter effect was checked thanks to a next experiment (Table 4). Details on the methods and statistics can be found in the text. n°: number; a = n° levels 2 + 3 + 4/ n° levels 1 + 2; s: second; min: minute
Social relationships
Ants belonging to a same colony present no aggressiveness towards one another. Drugs may affect this normal, peaceful social relationship. For examining potential social aggressiveness induced by oxybutynin, for ants under normal diet as well as for those under the drug consumption, five dyadic encounters were performed on ants of each two colonies (10 encounters in total). Each encounter occurred in a cylindrical cup (diameter = 2 cm, height = 1.6 cm), the borders of which having been slightly covered with talc to prevent escaping. During each of these encounters, one ant of the pair was observed during 5 min and its behavior towards the other ant was characterized by the numbers of times it did nothing (level 0 of aggressiveness), touched the other ant with its antennae (level 1), opened its mandibles (level 2), gripped and/or pulled the other ant (level 3), tried to sting or stung the other ant (level 4) (Figure 2E). The numbers of these behaviors obtained for the two colonies were added (Table 3, line 2). The sums obtained for ants under oxybutynin diet were compared to the sums obtained for ants under the normal diet by using the non-parametric χ² test.36 The ants’ level of aggressiveness was also assessed by a variable ‘a’, which equaled the number of aggressiveness levels 2+3+4 divided by the number of levels 0+1.
Cognition
To assess this trait, 15 ants of each colony were deposited in an own apparatus (Figure 2G), i.e.a tray (15 cm x 7 cm x 4.5 cm) into which two duly folded pieces of white extra strong paper (Steinbach ®, 12 cm x 4.5 cm) had been inserted in order to manage a twists and turns path between a small 2 cm long area in front of that difficult path and a larger 8 cm long area beyond the path. The 15 ants were set all together in the small area. After that, the ants still in front of the difficult path and those having reached the large area beyond it were counted after 2, 4, 6, 8, 10 and 12 min. The numbers obtained for the two colonies were added (Table 3, line 3). For ants counted in front as well as beyond the difficult path, the six sums obtained for ants consuming oxybutynin were statistically compared to those previously obtained for ants under normal diet using the non-parametric Wilcoxon test.36
Escaping behavior
For each two colonies, six ants were enclosed under a reversed polyacetate cup (h=8 cm, bottom diameter=7 cm, ceiling diameter=5 cm) placed on the foraging area. The lower part of the inner surface of the glass was slightly covered with talc to prevent the ants climbing. The ants were introduced into this enclosure through a hole (diameter = 3 mm) made in its ceiling. To assess the ants’ escaping behavior, a notch (3 mm height, 2 mm broad) had been made in the rim of the bottom of the cup so that the ants had the possibility of escaping (Figure 2F). The ants’ escaping score was established by counting those escaped after 2, 4, 6, 8, 10 and 12 min. The numbers obtained for the two colonies were added (Table 3, line 4). The six sums obtained for ants consuming oxybutynin were compared to those obtained for ants living under normal diet by using the non-parametric Wilcoxon test.36
Conditioning acquisition, memory
For colony C, and later on for colonies A and B, at a given time, a green hollow cube was placed above the entrance of the sugar water tube. Since that time, the ants underwent operant visual conditioning. The use of colony C was imperative since after individuals acquire conditioning to a cue, they are no longer naive for that cue, and no assessment of their conditioning ability can be made. The hollow cube under which ants could go was build in strong green paper (Canson®), the wavelengths reflection of which has been previously determined.38 From the time of the cube deposit, the ants were tested first in the course of their conditioning acquisition, then after the cue removal, in the course of their loss of conditioning. The tests were performed in a Y-apparatus made of strong white paper, set in a tray the sides of which having been slightly covered with talc to prevent escaping. The floor of these Y-apparatus was covered with a thin paper changed between each test. Also, each Y-apparatus was provided with a green hollow cube in one of its branch, half of the tests being made with the cube in its left branch and the other half with the cube in its right branch. To make a test, 10 ants of each colony were individually tested in a Y-apparatus: each one was successively deposited in the area lying on front of the choice point, i.e. before the two branches of the apparatus. The ants’ first choice of one or the other branch of the Y-apparatus was recorded when the ant was beyond a pencil-drawn line indicating the entrance of the chosen branch (Figure 2H). Choosing the branch containing the green cube was considered as giving the correct response. After having been tested, the ant was kept into a polyacetate cup until 10 ants of its colony were tested, this in order to not testing twice the same ant. After the test, the 10 ants were returned into their foraging area, near their nest entrance. For each test, the choices of ants of colonies A and B were added (n = 10 ants x 2 colonies = 20 responses) and the successive numbers of correct responses obtained for these ants consuming oxybutynin were statistically compared to those obtained for the ants of colony C living under normal diet, by using the non-parametric Wilcoxon test.36 The proportions of correct responses given by ants over time were also calculated (Table 4).
|
Time (h) |
Normal diet (colony C) |
Diet with oxybutynin |
|
7 |
5 vs 5 ; 5 vs 510 vs 10= 50% |
5 vs 5 ; 3 vs 78 vs 12= 40% |
|
24 |
7 vs 3 ; 6 vs 413 vs 7= 65% |
5 vs 5 ; 5 vs 510 vs 10= 50% |
|
31 |
7 vs 3 ; 7 vs 314 vs 6= 70% |
5 vs 5 ; 5 vs 510 vs 10= 50% |
|
48 |
7 vs 3 ; 7 vs 314 vs 6= 70% |
5 vs 5 ; 4 vs 69 vs 11= 45% |
|
55 |
8 vs 2 ; 7 vs 315 vs 5= 75% |
5 vs 5 ; 4 vs 69 vs 11= 45% |
|
72 |
8 vs 2 ; 8 vs 216 vs 4= 80% |
4 vs 6 ; 6 vs 410 vs 10= 50% |
|
Cue removal |
||
|
7 |
7 vs 3 ; 7 vs 314 vs 6= 70% |
|
|
24 |
7 vs 3 ; 7 vs 314 vs 6= 70% |
|
|
31 |
7 vs 3 ; 7 vs 314 vs 6= 70% |
|
|
48 |
7 vs 3 ; 7 vs 314 vs 6= 70% |
could not be examined |
|
55 |
7 vs 3 ; 7 vs 314 vs 6= 70% |
|
|
72 |
7 vs 3 ; 7 vs 314 vs 6= 70% |
|
Table 4 Effect of oxybutynin on the ants’ conditioning ability and memorization
The ants’ conditioning acquisition was impacted by the drug. Since ants under oxybutynin could learn nothing, we could not assess their long-term memory. The present result was in agreement with that on ants’ cognitive abilities (Table 3, lines 3, 4). Methods and statistical results are given in the text. vs: versus; %: percentage
Adaptation to side effects of oxybutynin
An individual becomes adapted to a drug or a situation when over time he (it) less and less suffers from the side effects of the drug or situation. For studying potential adaptation to a drug or a situation, a trait impacted by them must be assessed after one or two days of their use and then again after several days of use and the results of these two assessments must be compared. In the present work, an ants’ trait largely impacted by oxybutynin consumption and easy to assess was locomotion and more precisely angular speed. This trait was thus again assessed after 7 days of oxybutynin consumption, just like it had been assessed after one day of consumption (Table 5, line 1). The results obtained after 7 days of consumption were compared to the control ones and to those obtained after one day of consumption using the non-parametric χ² test.36
|
Adaptation to oxybutynin |
Normal diet |
1 day on oxybutynin |
7 days on oxybutynin |
|
Linear speed, mm/s |
12.2 (10.6–13.3) |
9.6 (8.2–11.0) |
9.1 (8.2–10.5) |
|
Angular speed, ang.deg./cm |
137 (119–146) |
150 (133–17) |
184 (166–207) |
|
Dependence on oxybutynin |
N° of ants on the sugar water |
N° of ants on the sugared oxybutynin |
|
|
after 9 days of consumption |
Colony A: 12 Colony B: 1 |
Colony A: 20 Colony B: 19 |
|
|
|
=25% |
|
=75% |
Table 5 Adaptation to side effects of oxybutynin and dependence on its consumption
Explanation about the methods and results of statistical analysis can be found in the text. Briefly, the ants did not adapt themselves to the side effect of the drug on their locomotion (i.e. to the induced increase of angular speed) (line 1). The ants became dependent on oxybutynin consumption: they preferred sugar water containing the drug than such water free of the drug (line 2). mm/s: millimeter per second; ang.deg./cm: angular degree per centimeter; N°: number; %: proportion
Habituation to wanted effects of oxybutynin
An individual becomes habituated to a drug or a situation when over time he (it) less and less perceives the wanted effect of this drug or situation. To study habituation, a trait improved by the drug or the situation should thus be assessed one or two days after their use as well as after several days of use, and the two results should be compared. In the present work on oxybutynin, none of the ants’ examined trait appeared to be improved by the drug. Consequently, habituation to oxybutynin consumption could not be examined.
Dependence on oxybutynin consumption
An individual becomes dependent on a drug or a situation when he (it) wants going on using this drug or situation and enjoys doing so, and can only live with them after having used them for some time. In the present work on oxybutynin, dependence on its consumption was examined after the ants had consumed it during 10 days. At that time, for colonies A and B, 15 ants were deposited in a tray (15cm x 7cm x 5cm) which contained two cotton-plugged tubes (h = 2.5 cm, diam = 0.5 cm), one filled with sugar water, the other filled with the sugar solution containing the drug delivered over the experimental work. The tube containing the drug was located on the right in the tray of one colony, and on the left in the tray of the other colony (Figure 2I). During 15 minutes, at the end of each minute, the ants of each colony approaching the entrance of each tube were counted, and the 15 corresponding counts obtained for each two colonies were added (Table 5, line 2). These totals allowed establishing the proportion of ants which chose the solution free of drug and that containing the drug. Also, the two obtained sums of counts were compared to those expected if the ants randomly approached the two presented tubes, using the non-parametric χ² goodness-of-fit test.36
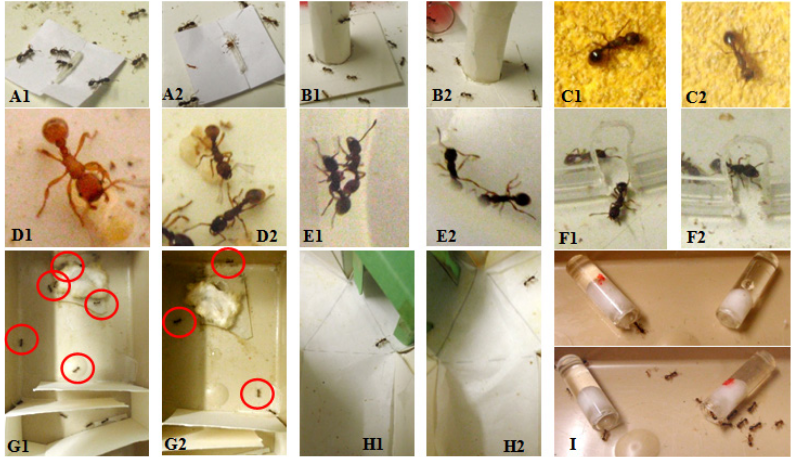
Figure 2 Some views of the experiments. 1: ants under normal diet; 2: ants under a diet with oxybutynin. A: ants’ orientation to a tied nestmate, obviously affected by the drug consumption. B: ants coming onto an unknown apparatus, also under drug consumption. C: ants walking on a rough substrate and less well perceiving its uncomfortable character under oxybutynin consumption. D: an ant under normal diet carrying a larva, and two ants under the drug diet disregarding a larva. E: two ants under normal diet staying side by side, on the contrary of two ants under the drug diet. F: an ant under normal diet escaping from an enclosure, and an ant under oxybutynin diet unable to do so. G: photos taken two minutes after the end of the experiment; more ants under normal diet could cross a twists and turns path than ants consuming oxybutynin. H: ants trained to a hollow green cube tested in a Y-apparatus provided with such a cube, the ants under normal diet giving the correct response but not the ants under the drug diet. I: ants under oxybutinin diet since 9 days preferring a sugar solution containing the drug (red dot) than a drug-free solution, having thus become dependent on this drug consumption.
Decrease of the effect of oxybutynin after its consumption was stopped
This decrease was studied after the ants had consumed oxybutynin during 12 days. The experimental protocol was similar to that used in previous studies [e.g.33–35], though here, we assessed only the ants’ angular speed, the trait the most impacted by the drug and the most easily assessed in a short time. The ants were provided with a fresh solution of the drug 12 hours before weaning. After these 12 hours, the ants’ angular speed was assessed just like it had been after one and seven days of consumption except that 20 instead of 40 ant trajectories were analyzed to be able to complete the assessments over the experiment. Indeed, after the assessment at t = 0h, weaning started: the sugared solution containing the drug was replaced by a usual normal sugared solution, and from this time, the ants’ angular speed was assessed every two hours until it became similar to that observed for ants under normal diet. The numerical results of this study are given in Table 6 and illustrated in Figure 3. The distributions of the angular speed values obtained over time after weaning were compared to that obtained at t = 0 as well as to that obtained for ants under normal diet by using the non-parametric χ² test for independent samples.36 The resulting P values (Table 6) were adjusted for multiple comparisons using the Benjamini-Hochberg procedure39 after having chose a false discovery rate (FDR) of 0.05. For calculation of the adjusted P values, < 0.001 was considered as = 0.001 and intermediate P values such as X1<P<X2 were used as P = (X1-X2)/2.
|
Time (h) |
Angular speed |
Statistics |
|||||
|
versus t = 0 |
versus control |
||||||
|
χ² |
df |
P |
χ² |
df |
P |
||
|
t = 0 |
188 (171–209) |
||||||
|
Weaning |
|||||||
|
2 |
184 (150–198) |
0.6 |
3 |
0.83 |
14.24 |
2 |
<0.005 |
|
4 |
160 (131–169) |
8.89 |
2 |
0.019 |
7.29 |
2 |
0.083 |
|
6 |
154 (130–167) |
12.15 |
2 |
0.005 |
5.91 |
2 |
0.083 |
|
8 |
153 (135–172) |
15.26 |
2 |
<0.005 |
3.53 |
2 |
0.19 |
|
10 |
136 (117–149) |
12.91 |
1 |
0.005 |
0.09 |
2 |
0.96 |
|
Control |
137 (119–147) |
|
|
|
|
|
|
Table 6 Decrease of the effect of oxybutynin after weaning
The effect of oxybutynin rapidly decreased after weaning, with the occurrence of two very quick decreases, a first one between 2 and 4 hours after weaning, a second one between 8 and 10 hours after weaning. The effect completely vanished in 10 hours. Such a decrease of effect after weaning accounted for the development of the ants’ dependence on the drug consumption (Table 5, lower part). The experimental protocol and statistical processing are described in Material and Methods; the results are graphically presented in Figure 3. t: time
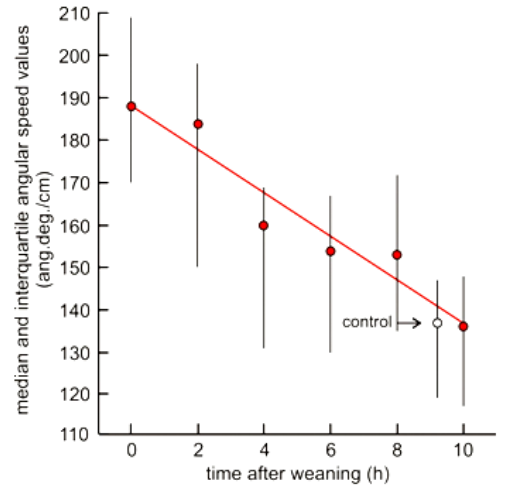
Figure 3 Decrease of the effect of oxybutytine after weaning. The graph plots the median and quartiles of the ants’ angular speed with the running time. The effect of oxybutynin quickly decreased after weaning: it entirely vanished in 10 hours, and moreover, very quickly decreased between 2 and 4 hours as well as between 8 and 10 hours after weaning. This kind of decrease accounted for the ants’ development of some dependence on the drug consumption (Figure 2I). Details and statistics are given in the text and in Table 6.
Food consumption, general activity
Oxybutynin impacted these three physiological traits (Table 1). Ants consuming this drug eat less meat and drunk less than ants living under normal diet, and this was significant (N=5, T=-15, P=0.031). Quite the reverse, ants consuming oxybutynin were more active, and observation showed that they were more excited, nervous, than those maintained under normal diet. This was however at the limit of significance (N=5, T=+14, P=0.063) because the effect was not yet effective during the two first days of the drug consumption. We checked if the three here pointed impacts of oxybutynin were really due to the drug consumption by giving it to the ants of another colony (a colony different from those mentioned in the subsection ‘Collection and maintenance of ants’) and observing these ants after two days: they obviously scarcely eat and drunk, and erratically walked at any time.
Linear and angular speeds
Oxybutynin impacted the ants’ locomotion (Table 2, lines 1, 2). While consuming this drug, the ants walked erratically, sinuously, often changing their direction of movement. The ants’ linear speed was significant lower (χ²=24.03, df=2, P<0.001) and their angular speed significantly higher (χ²=6.02, df=2, P < 0.001) than those of ants under normal diet. This result was in agreement with the previous one on the ants’ activity.
Orientation
Oxybutynin largely affected this trait (Table 2, line 3; Figure 2A). While ants under normal diet correctly oriented themselves towards a tied nestmate, those consuming the drug did so poorly. The difference was highly significant (χ²=20.20, df=2, P < 0.001) and could be due to the impact of the drug on the ants’ locomotion or could result from a lower olfactory perception of ants consuming oxybutynin, the later presumption being checked in following experiments.
Audacity
Oxybutynin appeared to slightly affect this ethological trait (Table 2, line 4; Figure 2B). Ants consuming the drug walked more frankly on the unknown apparatus and were more excited than ants living under normal diet. However, the difference between the two kinds of ants was not significant (N=4, T=9, P=0.125), due to the smallness of the sample. Nevertheless, this result confirmed the previously observed high activity and nervousness of ants consuming oxybutynin.
Tactile (pain) perception
This physiological trait was affected by oxybutynin consumption (Table 2, lines 5, 6; Figure 1C). While, on a rough substrate, ants under normal diet moved very slowly, with difficulty, sinuously, touching the substrate with their antennae, those consuming the drug moved more frankly, more rapidly, less sinuously, maintaining as usual their antennae above the substrate. The by latter ants obviously less perceived the uncomfortable character of the substrate than the former ants. This difference was highly significant: linear speed: χ²=19.76, df=1, P < 0.001; angular speed: χ²=12.00, df=1, P < 0.001. Oxybutynin reduced thus the ants’ perception, what has been presumed in the subsection relative to orientation and what was again examined in the course of two following experiments (subsections on brood caring and social relationships).
Brood caring
This ethological trait was largely impacted by oxybutynin consumption (Table 3, line 1; Figure 2D). Under normal diet, the ants soon perceived the larvae removed from the nest, hold them between mandibles and quickly re-entered them. While consuming the drug, the ants very poorly perceived the larvae, they even walked on them and went on their way erratically moving. They almost never stopped aside a larva, scarcely hold and transported it. This difference of behavior was obvious to the observer, and statistically significant (N=6, T=-21, P=0.016). This absence of care could be explained by a decrease of perception, as well as by an abnormal excitability, nervousness and walking, two facts previously pointed out (subsections relative to tactile perception, orientation, general activity, and linear and angular speeds).
Social relationships
The social relationships between nestmates were impacted by oxybutynin consumption (Table 3, line 2; Figure 2E). Under normal diet, the nestmates often stayed side by side doing nothing or touching themselves with their antennae. They also seldom slightly opened their mandibles. While consuming oxybutynin, the nestmates rarely stayed side by side (we never succeeded in photographing such side by side nestmates) but stayed apart. When exceptionally approaching a nestmate, they contacted it with their antennae, as usual, but also often widely opened their mandibles. The difference of behavior between ants maintained under the two kinds of diet was highly significant: χ²=38.81, df=2, P < 0.001. Such a difference may be due to a lower olfactory perception in ants consuming the drug (as previously presumed: see the subsections relative to orientation, tactile perception and brood caring), as well as to their almost continuous moving (as revealed by their high general activity: see the subsection relative to this activity).
Cognition
This ethological trait was affected by oxybutynin consumption (Table 3, line 3; Figure 2G). Over the 12 experimental minutes, more ants consuming the drug were counted in front of the twists and turns path than were ants living under normal diet, and in the same way less ants consuming the drug were counted beyond this path than were ants living under normal diet. These differences were significant (in front of the difficult path: N=6, T=+21, P=0.016) or at the limit (beyond that path: N=4, T=10, P=0.063). This is probably partly due to the erratic displacement of the ants (they very often came back on their way), but may also be caused by some impact of the drug on the ants’ cognitive abilities, a presumption checked in the two next experiments.
Escaping behavior
This ethological trait, influenced by the individual physiological state, was impacted by oxybutynin consumption (Table 3, last line; Figure 2E). This was obvious to the observer and statistically significant (N=6, T=-21, P=0.016). Under normal diet, the ants walked for a short time all around the bottom of the enclosure, then walked essentially along its rim. They thus rather rapidly found the exit and went out. While consuming oxybutynin, the ants walked erratically all around the bottom of the enclosure, then also along its rim but being excited, nervous, in state of stress. They did not or infrequently perceive the small exit in the rim. Only 4 ants consuming the drug could escape in the course of the 12 experimental minutes, while 9 ants not consuming the drug could do so over the same time period. The difference of behavior between the ants living under one or the other kind of diet resulted thus from the state of stress and excitability of those consuming oxybutynin. However, the difference may also be due to some impact of the drug on the ants’ cognitive abilities, a presumption previously made (see the subsection on cognition) and further checked in the next experiment.
Conditioning acquisition, memory
This ethological and physiological trait was largely impacted by oxybutynin consumption (Table 4, Figure 2H). The ants of colony C maintained under normal diet easily acquired visual conditioning, reaching a final score of 80%. On the contrary, the ants of colonies A and B maintained under a diet with oxybutynin never acquired such a conditioning. The difference between the two kinds of ants was significant (N=6, T=-21, P=0.016). The drug affected thus the ants’ cognitive ability, a presumption we had in the course of the two previous experiments. We can state that the short-term ants’ memory was impacted since they never memorized the presented visual cue, but we could not examine their long-term memory since they learned nothing. However, they went on going to their nest entrance, food sites, and cemeteries, and still accepted their nestmates. Therefore, we can presume that oxybutynin did not affect the remembering of what had been memorized before its consumption.
Adaptation to side effects of oxybutynin
The ants did not adapt themselves to the side effect of oxybutynin on their locomotion. This was obvious to the observer and was confirmed by the numerical results (Table 5, first line). After 7 days of drug consumption, the ants’ linear speed was still lower and their angular speed still higher than those presented under normal diet (linear speed: χ²=29.68, df=2, P < 0.001; angular speed: χ²=21.00, df=2, P < 0.001). Even more, the side effect of oxybutynin seemed to increase over the time of its consumption. While the ants’ linear speed after 7 days of consumption was similar to that after 1 day (χ²=1.68, df=2, 0.30 < P < 0.50), their angular speed after 7 days of consumption was higher than that after 1 day (χ²=13.06, df=2, 0.001 < P <0.01). To the observer, the ants consuming oxybutynin stayed nervous, excited, nearly always active, and erratically moving all over the time of their consumption, and this even seemed to increase over time.
Habituation to wanted effects of oxybutynin
Having detected no favorable effect of oxybutynin on the ants, the assessment of this trait was unrelevant.
Dependence on oxybutynin consumption
The ants became dependent on oxybutynin consumption (Table 5, second line; Figure 2I). Among 52 visits, 13 were for the tube free of the drug and 39 for the tube containing oxybutynin. This corresponded to 25% of ants’ choices of pure sugar water and 75% of choices of sugared solution of the drug. The difference was significant: χ²=5.90, df=1, 0.01 < P < 0.02. During the experiment, we observed that the ants choosing the tube containing the drug were essentially old ones, i.e. those collecting sugar water and having thus the most consumed the drug. This observation confirmed that ants’ consumption of oxybutynin leaded them to dependence on that drug.
Decrease of the effect of oxybutynin after its consumption was stopped
After weaning, the decrease of the impact of the ants’ previous oxybutynin consumption on their angular speed was graphically best described by a linear function (median angular speed = 188.14 – 5.13 h; R2=0.93), however with a quick decrease between 2 h and 4 h followed by a slowing down from 4 h till 8 h and again a quick decrease between 8 h and 10 h after weaning, the effect of the drug disappearing in 10 h. Numerical results and statistics are given in Table 6, and the decrease is illustrated in Figure 3. In detail, at t=0, the ants’ angular speed was similar to that observed after 7 days of oxybutynin consumption (Tables 5 & 6), what confirmed the ants’ non adaptation to the side effect of the drug. Two hours after weaning, the ants’ angular speed was still similar to that presented at t=0 and different from the control one. Four hours after weaning, the ants’ angular speed already became somewhat different from that at t=0 (P=0.02) and nearing that of the control one (P > 0.05) the level of which was reached 10 h after weaning. The overall quick decrease of the effect of oxybutynin was in agreement with the ants’ development of dependence on the drug consumption and with the medical advice to consume per day 2 to 3 tablets containing 5mg of the drug.
Urinary incontinence is mostly treated thanks to oxybutynin. Here, we examined, on ants as models, the side effects of this drug. We found that oxybutynin diminishes the ants’ food consumption and increases their general activity. This agrees with the adverse side effects of anorexia, restlessness and anxiousness in humans consuming this drug, these effects being reported in the instructions for use of this medication.We also found that oxybutynin affected the ant’s locomotion, orientation, audacity and tactile perception. This recalls the prolongation of reaction time observed in healthy humans under oxybutynin or tolterodine as measured by electromyographic activity of the flexor digitorum superficialis muscle.40
Moreover, oxybutynin was found to impair the ants’ cognition, learning and short-term memory. In healthy aged humans, it has been shown since a long time that a double-blind administration of 5–10 mg oxybutynin 90 minutes before testing significantly altered their attention, concentration, reaction time, learning and memory.41 Contrary to solifenacin 10 mg, the use of oxybutynin 10 mg was associated with a decline in cognitive functions (power and continuity of attention, working memory, episodic memory, speed of memory and alertness).42 A difference in cognitive function between pre-treatment and post-treatment, scored by using a Mini Mental State Examination test (MMSE), was however not detected in a small group of geriatric patients treated with oxybutinin for OAB.43 Headache, dizziness, somnolence and even anxiety and nervousness4,13 are also reported. More rarely, hallucinations, disorganized speech and behavior have been observed in young people.44 Sleep impairment was also demonstrated45 as well as impairments of accuracy of perception, concentration and vigilance.4 With adverse effects corresponding to those observed in humans, ants appear to be a valuable biological model for testing substances intended for human care.
In addition, we found that the ants’ social relationships were impaired by oxybutynin consumption. In humans, this effect may correspond to psychotic disorders such as paranoia, sometimes reported.11 We also showed that ants did not adapt themselves to the effect of oxybutynin on their locomotion, that they became dependent on its consumption, and in agreement with this last point, we found that the effect of the drug on their locomotion rapidly decreased, totally vanishing in 10 hours.This can explain their dependence on the drug. The short half-life of oxybutynin IR taken orally by humans (2-3 hours4) is expected to lead to some dependence on this drug.
Examining potential habituation to the wanted effect of the drug was of course irrelevant in ants. On the contrary, it is important to examine in humans if the efficiency of oxybutynin stays unaltered over its consumption or if some habituation to its effect occurs over time, what would lead to an increase of its consumption. Compared to other used antimuscarinics such as darifenacin, trospium and tolterodine, oxybutynin appears to produce a high benefit against AOB symptoms43 what can be accounted for its high binding affinity for M3 receptors,5 the most important acetylcholine receptors for detrusor contraction, and what explains why oxybutynin is still used. However, this affinity for M3 receptors leads to significantly more harmful effects such as dry mouth and eyes, constipation, dizziness and dyspepsia, and to significantly more treatment discontinuation.43 Moreover, oxybutinin has a low differential affinity for M3 vs M1 receptors (pKi ratio of receptor-binding affinity estimate: 1.56,46), and among the other anticholinergics, it penetrates the more readily the blood-brain barrier (BBB),47 what explains why this drug can lead to more severe negative effects on the CNS functioning, including significant EEG activity changes,48 than the other antimuscarinics.
A meta-analytic study of literature showed that among seven muscarinic receptor antagonists used for treating overactive bladder (oxybutynin, tolterodine, darifenacin, fesoterodine, solifenacin, propiverine and trospium chloride), orally administrated ≥10 mg oxybutynin IR demonstrated the worst adverse events, while the use of solifenacin and trospium showed the less adverse profiles.7 Researchers and practitioners are thus looking for alternative drugs or treatments allowing caring urinary incontinence. Most of these drugs are antimuscarinics, which were for a long time qualified as the only treatment with undisputed effectiveness.49 Double-blind studies showed that ca 50% of elderly patients achieved restoration of continence after 12 weeks of solifenacin treatment as compared to ca 30% under placebo and that a 5 mg daily dose showed no more dry mouth, constipation and urinary tract infection than placebo.50 A review of randomized controlled trials showed that solifenacin was not inferior to tolterodine in terms of efficacy, with comparable overall adverse effects, although with significantly higher rates of constipation and blurred vision.51 A randomized double-blind comparison of the effects of solifenacin and mirabegron on OAB patients showed no significant differences after 12 weeks’ treatment, although a lower dry mouth incidence was observed with mirabegron.52
Darifenacin, a large molecule which has the highest selectivity for M3 vs M2 (a pKi ratio of 59.2) and vs M1 receptors (pKi ratio of 9.3)6,46,47 has been proved to not impair cognition,53 memory, cardiac and visual functions.54,55 Trospium chloride is used in Europe (Germany) since the year 1999. Though it presents a M3/M2 pKi ratio of 1.3 and a higher affinity for mucosal than for detrusor receptors,6,46 this drug appears to be efficacious in elderly patients.56 Contrary to the other antimuscarinics which are tertiary amines, it is less lipophilic and highly charged. Consequently, it does not readily pass the blood-brain barrier47 and thus, as expected, contrary to oxybutynin, it has no CNS side effects such as accuracy of perception, concentration and vigilance,4 dizziness, vertigo, sommnolence and visual disturbances.57 Moreover, unlike many other antimuscarinics, being poorly metabolized,46 trospium did not show harmful drug interactions with concomitant medications, what is advantageous for elderly patients.58 Note that, as a blood-brain barrier is absent in insects, it would be irrelevant to use ants as models for comparing CNS side effects of trospium in ants and humans.
Mirabegron, a selective β3-adrenoreceptor agonist relaxing the detrusor muscle, is a drug with a different mode of action. It was approved for medical use in the USA and in the European Union in 2012. In 2017, it was already the 191st most commonly prescribed medication in the United States (Wikipedia). Mirabegron diminishes more the bladder pressure and number of microcontractions than oxybutynin.59A Bayesian mixed treatment comparison based on peer-reviewed articles on the treatment of overactive bladder enabled to compare the effectiveness of six muscarinic receptor antagonists (oxybutynin, tolterodine, darifenacin, fesoterodine, solifenacin and trospium chloride), between them and against mirabegron. Solifenacin was found to diminish the more the number of daily micturitions, followed by trospium and mirabegron. Solifenacin also was found to be the more effective concerning number of episodes of incontinence and, with oxybutynin to reduce the more the number of urgency incontinences, followed by trospium. No any antimuscarinic better reduced dry mouth occurrences than mirabegron (a non anticholinergic drug) while oxybutynin IR was the worst. Moreover, mirabegron scored the best concerning constipation.60 Like with oxybutynin, the amplitude and duration of the rhythmic bladder contractions significantly decreased with mirabegron; however their interval was significantly elongated.61 Mirabegron also enabled to attain a longer persistence of treatment than oxybutynin.62 A survival analysis of time to treatment discontinuation showed that mirabegron very significantly ensured a longer persistence of treatment (i.e. without withdrawal) than 9 antimuscarinics used for overactive bladder treatment, including oxybutynin IR and ER.63 No more evidence of increased cardiovascular risk was found for mirabegron as compared to oxybutynin,64 solifenacin or tolterodine.65 Thus, mirabegron appears to be a valuable substitute to oxybutynin.
We should add that in conjunction with an alpha-blocker such as tamsulosin, the use of tolterodine extended-release (ER)66 or solifenacin67 improved urinary storage symptoms for patients with both OAB and benign prostatic hyperplasia, although dry mouth was more frequently reported when tamsulosin was combined with tolterodine (in 21% of 225 patients) than with solifenacin (in 4% of 76 patients). An attempt to relief OAB symptoms without a drug therapy, thus without unwanted effects, is based on a behavioral treatment i.e. the learning of bladder-control by pelvic floor muscle training and urge suppression techniques. This was found at least as effective as the use of an oxybutynin ER therapy.68 However, this therapy requires that the patient is motivated, is ready to use muscle and mental techniques, and accept to maintain these behavioral changes. A long term acupuncture treatment could also be a non-drug alternative to an oxybutynin therapy.69 No doubt that research will be going on to find efficient alternative medical procedures without adverse effects for treating OAB symptoms.
To conclude, we estimate that using ants as models to reveal side effects of a drug was a rapid, easy and rewarding method. The ants’ consumption of oxybutynin at a dose comparable to that taken by humans had adverse effects on their food intake, locomotion activity, sensitivity, cognition, and social relationships. Neither dependence on the drug nor adaptation to its side effects was observed. Therefore, from a medical point of view, we advice practitioners cautiously using oxybutynin, essentially when treating children who are in their learning period of life. Practitioners should also be attentive to their patients’ potential hyperactivity, their sensory perception, social relationships, cognition, learning and memory, as well as to their potential dependence on the drug consumption. We also suggest examining the patients’potential habituation to the wanted effect of this drug. Until now, the use of mirabegron for OAB treatment appears to provide the best balance between efficiency and side effects and thus to be the best alternative to oxybutynin.
None.
This was a self-supported study and we declare that we have no conflict of interest as for the use of any AOB treatment.
None.

©2020 Cammaerts, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.