MOJ
eISSN: 2574-9722


Review Article Volume 7 Issue 2
Research Laboratory in Microbiology and Microbial Biotechnology, University of Science, Techniques and Technology of Bamako, Mali
Correspondence:
Received: February 16, 2022 | Published: April 29, 2022
Citation: Koumaré Y, Babana AH, Bah A, et al. Bacteria isolated from Niger River water in Bamako showed multi-resistance to antibiotics. MOJ Biol Med. 2022;7(2):71-74. DOI: 10.15406/mojbm.2022.07.00168
In Bamako; infectious diseases caused by antibiotic resistant bacteria are major public health problem. Because of the ability of these bacteria to resist to one or more antibiotics, these diseases have been more difficult to treat. Because of antibiotic contamination derived from human activities, rivers become the reservoir for the dissemination of antibiotic resistant bacteria. The use of water containing antibiotic resistant bacteria increased the health risks associated with the waterborne bacteria in animals and humans. The aim of this study is to investigate the presence of antibiotic resistant bacteria from the Niger river water in Bamako rivers. A total of 177 pathogenic bacterial strains were isolated. Among the 177 bacterial isolates, thirty-one (31) were isolated as Staphylococcus aureus, sixty-four (64) were isolated as Salmonella sp. and eighty-two (82) as Escherichia coli. The results from this study showed that the Niger river in Bamako is exposed to high level of antibiotics compound which may lead the bacteria to develop mechanism of antibiotic resistant. This may be behind serious threats to the public health and environment.
Keywords: Antibiotic resistance, Water, Niger river, Bamako, Mali
River water is the most important resource for people and economic development; The social situation of a country is extended related to the availability as well as its distribution. Because of growing populations and industrial sectors, the transmission of waterborne infectious diseases becomes worrying for the population; in recent.1 The Niger River is a freshwater stream that divides the city of Bamako into two banks, right and left. It is subject to urban, agricultural and industrial pressure, and wastewaters ae directly discharged into the river without treatment.2 The composition of domestic wastewater can be extremely variable and will depend on three factors:
In addition, a number of important pathogenic bacteria, such as Coliforms, visible in polluted water are considered as indicators of fecal pollution.4 Escherichia coli and other fecal intermediate forms, all from the coliform group, are used as standard bacterial indicators of water quality since 1920.5 E. coli strains have been used as an effective indicator to assess freshwater quality.6 However, a number of pathogenic bacteria isolated from fresh water showed resistance to various antibiotics currently in use. So, drug resistance genes can be easily spread to other pathogens prevalent in the neighborhood and pose powerful health risks. Accordingly, it becomes important to monitor regularly coliform levels in the environment to provide insight into the state of water potability, warns of past analysis, and propose the way for the design of measures. corrective. These bacterial pathogens isolated from water are highly resistant and carry resistance genes and also spread them easily to other pathogens prevalent in the vicinity, hence pose powerful health risks.4 Antibiotic resistance is believed to be the leading cause of death worldwide.7 The massive use of antibiotics in all development sectors (clinical, agricultural and veterinary) would be an analog to the accumulated resistance of bacteria to these frequently used antibiotics.8 The transfer of resistance genes from the environmental bacteria to human pathogens is seen as the main risk for public health is that. That’s why, the aim of this study is to investigate the presence of pathogenic bacteria resistant to antibiotics in the Niger river water in Bamako. In this study, we evaluated and the emergence of antibiotic resistance bacteria and the implication of antibacterial resistance organism in the Niger river water in Bamako.
Sample collection
The Niger river water samples were collected in three periods: dry, rainy and cold season, from the Niger River at the level of the Martyrs bridge collector, a source of water pollution in Bamako. During two years from 2019 to 2020, three (3) water samples were collected per month, 10 days apart, from the Niger river during each season for a total of 54 samples. Samples were collected aseptically using Plastic containers sterilized with 70% (v/v) alcohol, rinsed three times with sample water before filling the sample container with the sample. The collected samples were protected from direct sunlight and transported in a cooler box containing ice packs to the laboratory for analyses. All samples were stored at 4oC and analyzed within 24 h of sample collection.
Bacterial Isolation and Identification
The river watersampleswere analyzed for the target bacteria using the standard methods for the examination of. Water and wastewater.9 According to,10 prior to analysis,the Niger river water samples were mixed to distributethe uniformly bacteria. Then, dilutionsof water samples from 10-2 to 10-6, were prepared according to.11 Fifty milliliters from each dilution replicate ofeachriver water sample was filtered using a 0.45μm diameter,cellulosic grid filter placed on the filter holder.10 To wet the filter paper, approximately 25ml of distilled water was first added. Selective media for eachpathogenicbacteria (Table 1), wereused and prepared according to the procedurerecommended by the manufacturer and sterilized byautoclaving at121°C, for 15 minutes.Treated membrane filters were aseptically transferred to 90mmPetridishes with the appropriate selective media:
Bacteria |
Media |
Incubation conditions |
Staphylococcus aureus |
Chapman afar |
37oC for 24hours |
Salmonella/Shigella |
Mac Conkey agar |
37oC for 24hours |
Escherichia coli |
Tryptone Bile X-glucuronide (TBX) agar |
44°C for 24hours |
Total coliforms |
Eosin Methylene Blue (EMB) |
37oC for 24h |
Fecal coliforms |
Eosin Methylene Blue (EMB) |
44oC for 24h |
Table 1 Media and incubation conditions used for the enumeration, and primary isolation of the indicated bacteria from Niger river water samples
Antimicrobial Susceptibility Test
In the present study, the antimicrobial susceptibility test was done on Mueller-Hinton agar (Merck, Germany) using the disk diffusion (Kirby Bauer’s) technique, performed according to Clinical and Laboratory Standards Institute (CLSI) guidelines to determine susceptibility of UTIs agents.12 The disks of antibiotic used comprised:Amoxicillin (10mg), Ceftriaxon, Gentamicin, Ciprofloxacin, Penicillin G, Erythromycin, Doxycycline and Cotrimoxazole. The antimicrobial susceptibility test was carried out on the 8 discs of antibiotics frequently used in Mali: Amoxicillin, Ceftriaxone, Gentamicin, Ciprofloxacin, Penicillin, Erythromycin, Doxycycline and Trimethoprim-Sulfamide. The test of antibacterial resistance of all strains of bacteria was performed using the Kirby-Bauer disc diffusion method.12 For Briefly, each pure overnight bacterial culture was suspended in physiological saline to have a standard turbidity of 0.5 McFarland, that was streaked on Mueller Hinton agar platesand incubated 37°C for 24hours.13 After overnight incubation, the inhibition zone around each disc was measured based on the interpretive standard of the CLSI used to define the bacterial isolatesas sensitive, intermediate or resistant to the antibiotic evaluated
Multiple Antibiotic Resistance Index
For each pathogenic bacterial isolate, a multiple antibiotic resistance index(MARI) wasdetermined by using the formula MARI =a/b,where a is the number of antibiotics to which the testisolate depicted resistance and b the total number ofantibiotics to which the test isolate has been evaluated forsusceptibility (Salikan et al. 2020).A high-risk environment where antibiotics are often used, is indicated by aMARI value of 0.2.14,15
statistical analyzes
The test of bartlett was used to determine the homogeneity of the variance of the collected data. Non homogenous data were log-transformed. These log-transformed datawere subjected to analysis of variance and comparison of means using protected LSD test (P≤0.05).1 The statistical package SAS (Version 9 – SAS Institute) was used for all analysis. Additionally, correlations between bacteria, multiple resistant antibiotics at different seasons, and variability were calculated using Pearson's correlation coefficient (at the 5% level of significance).16 Variation in resistance between types of seasons against eight antibiotics currently used in Bamako.
Bacterial strains isolated from Niger river water samples
In the monitoring period, 54 samples were collected in three seasons in nine rounds where samples were taken from June 2019 to April 2020. A total of 319 bacterial colonies were isolated in samples from the Niger River water in Bamako (Table 2). The isolated colonies, which depend on the season, are distributed as follows: 99 colonies isolated during the rainy season, 98 colonies in the cold season and 122 in the hot season (Table 2). More bacterial isolates were obtained in hot season than in cold and rainy seasons.
Seasons No. of samples |
Number of isolates |
|||||
Staphylococcus aureus |
Salmonella sp. |
Escherichia coli |
Total coliforms |
Fecal coliform |
||
Rainy |
18 |
10 |
21 |
24 |
23 |
21 |
Hot |
18 |
03 |
24 |
28 |
23 |
20 |
Cold |
18 |
18 |
19 |
30 |
23 |
32 |
Total |
54 |
31 |
64 |
82 |
69 |
73 |
Table 2 Table of bacteria isolated during the three seasons from June 2019 to April 2020
Of the 54 water samples, taken and analyzed over the three seasons; 32Staphylococcus aureus, 64 Salmonella sp., and 82 E. coli were isolated and tested for their resistance pattern against 8 commonly prescribed antibiotics (Amoxicillin, Ceftriaxone, Gentamicin, Ciprofloxacin, Penicillin, Erythromycin, Doxycycline and Trimethoprim-Sulfamide).
Susceptibility of isolated bacterial pathogens to antibiotics
The susceptibility of tested bacterial pathogensto antibiotics, presentedin Figure 1 and Table 3, showed that, apart from Staphylococcus aureus isolated from Niger River water samples taken during the cold season, all Salmonella sp., E. coli and Staphylococcus aureus isolated from water samples from all seasons were multidrug resistant (MAR). All pathogenic bacteria (Staphylococcus aureus, Salmonella sp., and E. coli) isolated from water samples from the Niger River in Bamako, and tested against a concentration of 10μg/ml of all the antibiotics used, show resistance to at least two antibiotics (Table 3). High antibiotic resistant bacteria were found to be abundant in hotseason (summer) compared to cold (winter) and rainy seasons.17 Fecal coliform counts were higher in cold season than in rainy and hot seasons (Figure 1). No significant difference in fecal coliform counts, was observed between hot and rainy season (Figure 1).18 show that antibiotic-resistant bacteria were abundant in river water collected in winter and summer. They also show that the antibiotic-resistant E. coli was at least one order of magnitude lower in summer than in winter.18 Most of the pathogenic bacteria, isolated in the Niger river water samples were highly resistant to ampicillin, gentamycin, erythromycin and oxacillin. At the same time, only one bacterium isolated from Niger river water in Bamako (during all seasons) was resistant to the concentration of Cotrimoxazole used. Moreover, Staphylococcus aureus isolated during the cold season show no resistance against ampicillin, gentamycin, penicillin, erythromycin and oxacillin and Cotrimoxazole at the concentration tested (Table 3). Our results show that the highest levels of antibiotic resistance in Niger river water for E. coli were found for Amoxicillin (91%), Penicillin (96%), and erythromycin (100%) which are older antibiotics. Kumar et al. (2020); who evaluate the occurrence of bacteria resistant to antibiotics, the antibiotic-resistant gene, and the concentration of metal in the river of Sri Lanka; show that: for older antibiotics, the percentage of resistance of E. coli is higher than for other antibiotics. In our study, the resistance for E. coliis higher than those obtained by19 who showed that the highest levels of antibiotic resistance in urban surface waters in Brazil for E. coli, were obtained for ampicillin with 27.7%, tetracycline with 27.7% and amoxicillin with 24.0%.The low or absence of resistance of bacteria to Trimethoprim-Sulfamide may indicate that in all seasons the river has been contaminated with low levels of Trimethoprim-Sulfamide, and indicates low use ofTrimethoprim-Sulfamideby human activity in Bamako.20
|
|
Number and (%) resistant to antibiotic tested |
MAR index |
||||||||
Seasons |
Resistance phenotypes |
N. tested |
AM |
CRO |
GMI |
CIP |
PEN |
ERY |
DOX |
SXT |
|
|
Staphylococcus aureus |
18 |
7 |
5 |
3 |
3 |
7 |
11 |
6 |
9 |
0.35 |
Hot |
Salmonella/Shigella |
19 |
17 |
3 |
5 |
1 |
19 |
17 |
15 |
9 |
0.51 |
|
Escherichia coli |
30 |
26 |
12 |
14 |
6 |
29 |
30 |
23 |
15 |
0.67 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Staphylococcus aureus |
3 |
0 |
2 |
0 |
1 |
0 |
0 |
0 |
0 |
0.13 |
Cold |
Salmonella/Shigella |
24 |
22 |
2 |
8 |
0 |
24 |
24 |
23 |
8 |
0.58 |
|
Escherichia coli |
28 |
21 |
1 |
3 |
1 |
27 |
27 |
23 |
14 |
0.59 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Staphylococcus aureus |
10 |
3 |
3 |
0 |
0 |
3 |
7 |
2 |
2 |
0.29 |
Rainy |
Salmonella/Shigella |
21 |
11 |
1 |
1 |
5 |
19 |
18 |
16 |
8 |
0.24 |
|
Escherichia coli |
24 |
20 |
8 |
0 |
4 |
22 |
22 |
15 |
10 |
0.58 |
Table 3 Antibiotic resistance among isolates of environmental resistance indicator bacterial species by sampling season
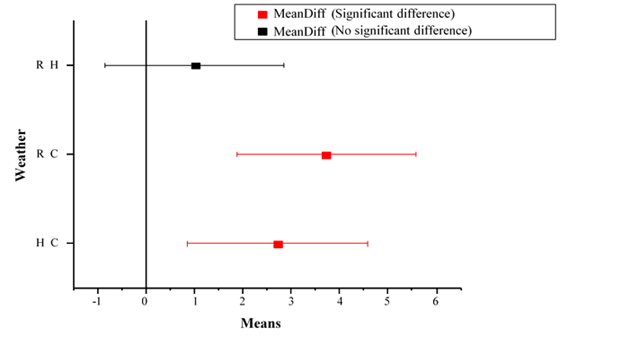
Figure 1 Effect of season in the occurrence of antibiotic resistant bacteria in Niger river water in Bamako. R; Rainy season, C: cold season and H: Hot season.
The municipal and industrial wastewater discharge into the Niger river water in Bamako can favorize the presence of residual antibiotics and antibiotic contamination in the river water.14,21 This can cause the emergence and development of bacteria resistant to antibiotics in the river water. In this study, the Niger River in Bamako was chosen to study the correlation between anthropogenic activity near the river with the apparition of antibiotic-resistant bacteria.22 indicate that the accumulation of municipal sewage with fecal wastesseems to be mainly responsible for deterioration of river water quality along with increased population of pathogenic bacteria resistant to antibiotics in the river. The increase of pathogenic bacteria resistant to antibiotics in the river water may affect negatively and seriously the public health and environment. In Bamako, the river plays an important asset for economic resources because of the many gardens, breeding site and hotels belonging to the residents as a source of income. The surrounding environment of agriculture, hotels and livestock can also become the cause of pollution of the river waters by antibiotics.15,23,24 The selection of Niger River was based to its the location in the urban area, often densely populated, affected by residential area, industrial activities and agricultural activities.
MAR Index Analysis
According to Table 3, a total of 174 bacteria out of 177 (81,4%) pathogenic bacteria isolated from the Niger river water in Bamakoare resistant to, at least, two antibiotics and have a MAR index (ou MARI) greater than 0.2.Similarly, all the pathogens isolated during the hot season and the rainy season have a MAR index greater than 0.2 (Table 3).23 isolating Antibiotic Resistant Bacteria from Rivers inTerengganu in Malaysia, obtained only 24 bacteria out of which 24 (100%) were resistant to at least two antibiotics. Those results show that the Niger river is highly polluted. Meanwhile, for the cold season, 51 out of 54 isolates show a MAR index greater than 20%. Based on the results, the bacterial pathogens isolated from the Niger rivers in Bamako were multi-drug resistant, as they were resistant to at least two antibiotics. A MAR index with a value greater than 0.2 indicates a “high risk” source of antibiotic contamination.25 The Niger river is located in the urban area which is affected by residential area, the industrial and agricultural activities. There are also small resident-owned gardens along the river. In this study, the samples obtained from the Niger River in Bamako were taken near the martyrs' bridge at the mouth of sewer C located between the Hotel Mariétou Palace and the Hotel Kampesky and several gardens located on the river bank. right, near the densely populated Bozola residential area. These results clearly show the emergence of antibiotic-resistant bacteria at the sampling points.
A total of 319 pure colonies of bacteria were successfully isolated in the water samples from the Niger River in Bamako. Among these bacteria; 32 Staphylococcus aureus, 64 Salmonella sp., and 82 E. coli were identified. Apart from 3 strains of Staphylococcus aureus, isolated during the cold season, all the pathogenic bacteria isolated from the Niger river water in Bamako have a MAR index greater than 0.2. The results obtained showed that most of the bacteria isolated during the 3 seasons showed multiple antibiotic resistance, indicating that the Niger River at Bamako could constitute a “high risk” source of antibiotic contamination.
None.
None.
The author has no financial or proprietary interest in any of material discussed in this article.

©2022 Koumaré, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.