MOJ
eISSN: 2471-139X


Research Article Volume 10 Issue 1
Department of Zoology, Mizoram University, India
Correspondence: Ganesh Chandra Jagetia, 10 Maharana Pratap Colony, Sector 13, Hiran Magri, Udaipur-313002, India
Received: August 30, 2023 | Published: October 5, 2023
Citation: Jagetia GC. Grapefruit flavonoid naringin protects V79 cells against the vinblastine-induced DNA damage in vitro. MOJ Anat Physiol. 2023;10(1):35-40 DOI: 10.15406/mojap.2023.10.00336
Vinblastine an antimitotic agent is used to treat hematological malignancies and in combination cancer chemotherapy. Vinblastine produces second malignancies in the survivors which can be reduced/prevented in cancer patients by intervention with natural products. Naringin a grapefruit bioflavonoid was tested for its chemoprotective activity in cultured V79 cells. The V79 cells were treated with 1, 2, 5, 10, 25, 50, 100, and 200 µg/mL of naringin 1 h before exposure to 10 µg/mL of vinblastine. The DNA damage was evaluated by micronucleus assay at 22 h after vinblastine exposure in mononucleate, binucleate, and trinucleate cells. Vinblastine treatment increased the frequency of micronuclei significantly in both the mononucleate and binucleate V79 cells whereas different concentrations of naringin significantly reduced the formation of vinblastine-induced micronuclei. A maximum reduction in micronuclei formation was recorded at 200 µg/mL naringin in both mononucleate and binucleate cells. The determination of cell proliferation indicates that naringin treatment impacted the cell proliferation, especially at 2 to 25 µg/mL which increased at 200 µg/mL in both the naringin and Naringin+Vinblastine groupsgroup as indicated by a rise in binucleate and trinucleate cell numbers. The present study indicates that naringin protects the V79 cells against vinblastine-induced DNA damage indicated by significant attrition in micronuclei formation and 200 µg/mL naringin was highly effective.
Keywords: V79 cells, vinblastine, naringin, micronuclei, DNA damage
Vinblastine is a bisindole alkaloid isolated from Madagascar periwinkle plant Vinca rosea (Catharanthus roseus) in the 1950s by the Canadian scientists Robert Noble and Charles Beer.1 It has been used to treat various cancers including Hodgkin's disease, Kaposi's sarcoma, certain types of lymphomas, testicular cancer, lung cancer, renal cell carcinoma, breast cancer, and choriocarcinoma, anaplastic large cell lymphoma either alone or in combination with other chemotherapeutic agents.2–7 Vinblastine therapy in cancer patients leads to encephalopathy, seizures, visual loss, bone marrow, gastrointestinal, renal, pulmonary, liver, and cardiac toxicity.7 This indicates the need to reduce the adverse effects of vinblastine treatment. The name naringin (Figure 1) finds its origin in the Sanskrit word Narangi which stands for oranges. The grapefruit contains approximately 10% of naringin in respect to its dry weight and 800 mg/L of naringin is present in one liter of grapefruit juice. The red grapefruit contains only 332 mg/L of naringin.8–9 The naringin is transformed into naringenin in the human gut by intestinal flora and it is an aglycone form of naringin.10 Several reports suggest that naringin acts as an antiviral, antiatherogenic, anti-inflammatory, antimutagenic, cardioprotective, cholesterol-lowering, neuroprotective, and radioprotective agent.11–20 The antimutagenic and genotoxic effects of naringin have been reported against doxorubicin, and bleomycin in vitro and in vivo.19–22 It also declines tumor formation in the forestomach of mice treated with benzo-a-pyrene.23 Naringin alleviated iron-induced biochemical stress in vitro and in vivo.24,25 It has been reported to reduce doxorubicin-induced cardiotoxicity without compromising the antineoplastic action of doxorubicin in tumor-bearing mice.15 Naringin protects against bleomycin-induced bone loss induced by sciatic neurectomy, dyslipidemia, lung fibrosis, and lipodystrophy in rats.26,27 A single dose of 16 g/kg and 1.250 mg/kg body weight naringin once daily for 13 weeks orally has been reported to be nontoxic in rats.28 Naringin is an effective treatment for obese patients because it reduces lipid profile.29 Therefore, the present study was undertaken to evaluate the protective effect of naringin (7-(2-O-(6-deoxy-alpha-L-mannopyranosyl)-β-D-gluco-pyranosyloxy)-2,3-dihydro-4',5,7-trihydroxyflavone) on vinblastine-induced DNA damage in cultured V79 cells.
Chemicals
The grapefruit bioflavonoid naringin (Figure 1) was procured from Across Organics, Geel, Belgium, and vinblastine sulfate was a kind gift by Eli Lilly and Company, Indiana, Indianapolis, USA. Fetal calf serum, minimum essential medium (MEM), L-glutamine, gentamicin sulfate, and cytochalasin-B were supplied by Sigma Chemical, St Louis, USA. Dimethylsulfoxide and other routine chemicals were requisitioned from Merck India, Mumbai.
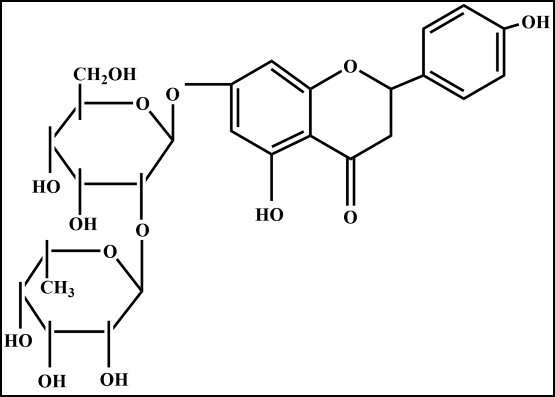
Figure 1 Chemical structure of naringin, 7-(2-O-(6-deoxy-alpha-L-mannopyranosyl)-β-D-glucopyranosyloxy)-2,3-dihydro-4',5,7-trihydroxyflavone.
Cell line and culture
Mycoplasma-free V79, Chinese hamster lung fibroblast cells were supplied by the National Center for Cell Sciences, Pune, India. The doubling time of V79 cells is about 16 to 20 h. V79 cells were grown in 25 cm2 Falcon (Beckton Dickinson, USA) culture flasks filled with 5 mL MEM containing 10% fetal calf serum, 1% L-glutamine, and 50 μg mL−1 gentamycin sulfate at 37°C in a CO2 incubator (NuAire, Plymouth, MN, USA) in a humidified atmosphere of 5% CO2 in 95% air.
Experimental
Usually, 5X105 V79 cells were inoculated into several 5 mL culture flasks and allowed to grow overnight. The flasks containing V79 cells were divided into the following groups.
Naringin: The cells of this group were treated with 0, 1, 2, 5, 10, 25, 50, 100, and 200 µg/mL naringin for 1 h.
Naringin+Vinblastine: This group of cells were exposed to 0, 1, 2, 5, 10, 25, 50, 100, and 200 µg/mL naringin for 1 h. The medium was removed and the cells were washed twice with phosphate buffered saline (PBS) before treatment with 10 µg/mL vinblastine.
Micronucleus assay
Six- hours after treatment with vinblastine the cells were washed with PBS and treated with 3 µg/mL cytochalasin-B to block the cytokinesis. Sixteen hours after cytochalasin-B treatment the cells were washed with PBS and dislodged from the culture flasks by trypsin-EDTA treatment for micronucleus assay.21 The micronucleus assay was carried out as described earlier with minor modifications.30 The cells from individual cultures were collected in prelabelled separate tubes, centrifuged, kept in hypotonic (0.75% ammonium oxalate) solution at 37°C and centrifuged once again. The cell pellet was disturbed and a small amount of Carnoy’s (3:1 methanol and acetic acid) fixative was added to fix the cells. The cells were centrifuged and suspended in a small volume of fixative and placed on the precleaned coded slides for blinded observation. The cells were stained in acridine orange in Sorensen's buffer (pH 6.4), mounted in the buffer and observed under a Carl Zeiss Photomicroscope III (Gottingen, Germany) fitted with epifluorescence system. The mononucleate cells, binucleate cells and trinucleate cells were counted in such a way so as to get 1000 binucleate cells per culture for the presence of micronuclei in three individual cultures. The data on cell proliferation was also collected. The results were confirmed by repeating the experiment.
Statistical analysis
The data were analyzed using nonparametric Kruskal-Wallis one-way ANOVA followed by Dunn’s post hoc test to test the significance between the treatments and within the group using Origin Pro 8.5 (Origin Lab Corporation, Northampton, MA, USA) statistical software.
The results are expressed as percent micronucleated cells ± standard error of the mean (SEM) and cell proliferation (%) in Tables 1 & 2 and Figures 2–5.

Figure 2 Alteration in the frequency of micronuclei in mononucleate V79 cells treated with different concentrations of naringin before exposure to 10 µg/mL vinblastine. NIN: Naringin alone.

Figure 3 Alteration in the frequency of micronuclei in binucleate V79 cells treated with different concentrations of naringin before exposure to 10 µg/mL vinblastine. NIN: Naringin alone.
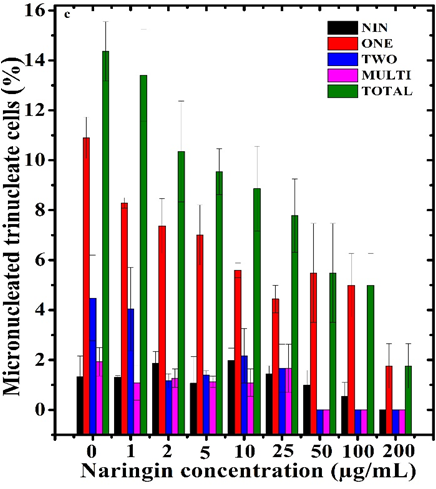
Figure 4 Alteration in the frequency of micronuclei in trinucleate V79 cells treated with different concentrations of naringin before exposure to 10 µg/mL vinblastine. NIN: Naringin alone.
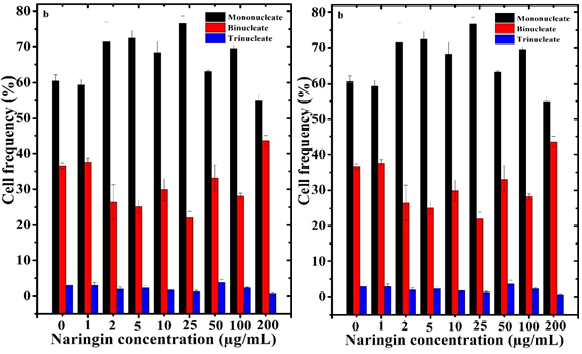
Figure 5 Cell proliferation kinetics of V79 cells treated with different concentrations of naringin before treatment with 10 µg/mL of vinblastine. a. Naringin alone and Naringin+vinblastine.
The frequency of mononucleate micronucleated cells declined concentration-dependently in the naringin treated group when compared to untreated cells (0 µg/mL). However, the difference among all concentrations of naringin was statistically non-significant (Table 1 and Figure 2–5). The vinblastine treatment alone significantly elevated the frequency of micronucleated mononucleate cells including the cells bearing two and more than two micronuclei (Table 1 and Figure 2). Exposure of V79 cells with 1, 2, 5, 10, 25, 50, 100, and 200 µg/mL naringin before vinblastine treatment significantly reduced the cells bearing one and two micronuclei depending on the naringin concentration, and maximum attrition in micronucleated cells was recorded at 200 µg/mL where this decline was 8.6 folds when compared to vinblastine treatment alone (Table 1).
The scoring of micronuclei in binucleate cells revealed that naringin treatment alone non-significantly reduced the spontaneous DNA damage in V79 cells at all concentrations (Table 1). Vinblastine treatment alone significantly elevated the frequency of binucleate cells with one, two, and greater than two micronuclei (Table 1). Treatment of V79 cells with naringin before vinblastine exposure led to a significant but dose-dependent decline in binucleated cells bearing one, two, and more than two micronuclei, and the greatest alleviation was detected at 200 µg/mL naringin where the micronuclei formation was 12.5 folds lower than vinblastine treatment alone. More than two micronuclei were completely absent in the 200 µg/mL naringin pretreated group (Table 1 and Figure 3).
The scoring of micronuclei in second division cells (trinucleate cells), indicated a significant rise in the micronucleated trinucleate cells after vinblastine treatment and treatment of naringin before vinblastine inoculation significantly reduced the trinucleate cells bearing one, two, and more than two micronuclei with a maximum depletion at 200 µg/mL naringin and this decline was 9.8 folds when compared to vinblastine treatment alone (Table 1 and Figure 4). The cells with two and more than two micronuclei were absent at 100 and 200 µg/mL naringin (Table 1 and Figure 4).
|
Naringin µg/mL |
Micronucleated cells (%)±Standard error of the mean |
||||||||||||||
|
Naringin alone |
Naringin+10 µg/mL vinblastine |
||||||||||||||
|
MNC |
BNC |
TNC |
Mononucleate cells |
Binucleate cells |
Trinucleate cells |
||||||||||
|
1 |
2 |
>2 |
Total |
1 |
2 |
>2 |
Total |
1 |
2 |
>2 |
Total |
||||
|
0 |
1.73± 0.36 |
1.90± 1.00 |
1.33± 0.83 |
8.66± 0.44 |
1.02± 0.15 |
0.18± 0.12 |
9.86±0 .57 |
8.30± 0.46 |
1.27± 0.09 |
0.53± 0.14 |
10.10 ± 0.51 |
10.90± 0.83 |
4.48± 1.72 |
1.93± 0.57 |
17.31± 1.96 |
|
1 |
1.57± 0.34 |
0.83± 0.12 |
1.31± 0.06 |
7.72± 1.61 |
1.23± 0.13 |
0.39± 0.13 |
9.35± 1.56 |
6.83± 0.32 |
1.37± 0.24 |
0.23± 0.14 |
8.43± 0.40 |
8.29± 0.21 |
4.04± 1.67 |
1.08± 0.69 |
13.41± 1.84 |
|
2 |
1.54± 0.19 |
1.23± 0.33 |
1.87± 0.47 |
6.94± 0.35 |
0.60± 0.09 |
0.27± 0.06 |
7.82± 0.32 |
6.70± 0.26 |
1.30± 0.32 |
0.07± 0.07 |
8.07± 0.07 |
7.37± 1.09 |
1.17± 0.28 |
1.27± 0.37 |
10.35± 2.02 |
|
5 |
0.53± 0.11 |
0.60± 0.06 |
1.07± 1.07 |
6.16± 0.79 |
0.49± 0.04 |
0.20± 0.01 |
6.86± 0.82 |
6.33± 0.33 |
1.20± 0.26 |
0.50± 0.25 |
8.03± 0.81 |
7.01± 1.20 |
1.40± 0.17 |
1.13± 0.22 |
9.54± 0.92 |
|
10 |
0.94± 0.04 |
1.10± 0.06 |
1.99± 0.48 |
4.64± 0.44 |
0.68± 0.17 |
0.17± 0.05 |
5.50± 0.06 |
5.97± 0.18 |
0.53± 0.09 |
0.20± 0.11 |
6.70± 0.06 |
5.59± 0.29 |
2.17± 1.09 |
1.09± 0.55 |
8.86± 1.70 |
|
25 |
1.13± 0.08 |
0.83± 0.13 |
1.44± 0.33 |
3.52± 0.35 |
0.37± 0.33 |
0.09± 0.01 |
3.98± 0.36 |
4.43± 0.29 |
0.37± 0.07 |
0.07± 0.03 |
4.87± 0.37 |
4.44± 0.55 |
1.67± 0.96 |
1.67± 0.96 |
7.78± 1.47 |
|
50 |
0.73± 0.26 |
0.63± 0.17 |
0.99± 0.60 |
2.86± 0.20 |
0.27± 0.05 |
0.13± 0.08 |
3.25± 0.22 |
3.00± 0.06 |
0.43± 0.09 |
0.03± 0.03 |
3.47± 0.09 |
5.49± 1.98 |
0 |
0 |
5.49± 1.98 |
|
100 |
1.05± 0.14 |
0.70± 0.06 |
0.55± 0.55 |
2.48± 0.23 |
0.20± 0.02 |
0.08± 0.04 |
2.76± 0.24 |
2.67± 0.09 |
0.53± 0.03 |
0.07± 0.03 |
3.27± 0.14 |
4.99± 1.27 |
0 |
0 |
4.99± 1.27 |
|
200 |
0.75± 0.02 |
0.37± 0.07 |
0.00± 0.00 |
1.00± 0.10 |
0.13 ±0.02 |
0 |
1.13± 0.12 |
0.60± 0.06 |
0.20± 0.06 |
0 |
0.80± 0.06 |
1.76± 0.89 |
0 |
0 |
1.76± 0.89 |
|
Kruskal Wallis p< |
NS |
NS |
NS |
0.002 |
0.01 |
NS |
0.002 |
0.001 |
0.01 |
0.05 |
0.002 |
0.02 |
0.02 |
NS |
0.01 |
Table 1 Effect of different concentrations of naringin on the vinblastine (10 µg/mL)-induced micronuclei formation in cultured V79 cells
MNC, mononucleate cells; BNC, binucleate cells; TNC, trinucleate cells
Cell proliferation
The naringin treatment arrested cell proliferation, especially at 2 to 100 µg/mL, and an increase was detected at 200 µg/mL however, the differences were non-significant (Table 2 & Figure 5). An almost similar pattern in cell proliferation was recorded for the Naringin+Vinblastine group (Table 2 & Figure 5)
|
Naringin µg/mL↑ |
Percent of different nucleated cells (Mean± Standard error of the mean) |
|||||
|
Naringin alone |
Naringin+10 µg/mL vinblastine |
|||||
|
Mononucleate |
Binucleate |
Trinucleate |
Mononucleate |
Binucleate |
Trinucleate |
|
|
0 |
60.45±0.77 |
36.94±0.35 |
2.61±0.42 |
60.43±1.75 |
36.52±0.79 |
3.05±0.98 |
|
1 |
54.53±1.56 |
42.23±1.74 |
3.23±0.14 |
59.37±1.40 |
37.58±1.12 |
3.05±0.75 |
|
2 |
50.87±2.59 |
44.92±1.95 |
4.21±0.84 |
71.53±5.36 |
26.43±4.87 |
2.04±0.51 |
|
5 |
54.9±4.53 |
40.75±3.41 |
4.36±1.11 |
72.46±2.16 |
25.14±1.89 |
2.39±0.51 |
|
10 |
55.80±3.18 |
41.25±3.38 |
2.95±0.63 |
68.34±3.22 |
29.89±3.05 |
1.77±0.19 |
|
25 |
55.98±4.06 |
40.75±3.33 |
3.27±0.95 |
76.64±2.02 |
22.07±1.71 |
1.29±0.37 |
|
50 |
50.17±1.21 |
45.02±0.89 |
4.27±0.52 |
63.06±4.39 |
33.14±3.57 |
3.80±0.85 |
|
100 |
52.23±1.53 |
45.62±1.69 |
2.15±0.34 |
69.46±0.95 |
28.14±0.78 |
2.39±0.20 |
|
200 |
54.20±4.15 |
44.45±3.87 |
1.37±0.34 |
54.93±1.75 |
43.61±1.43 |
0.67±0.33 |
Table 2 Effect of different concentrations of naringin on the frequency of different nucleated V79 cells treated with 10 µg/mL vinblastine
The adverse effects of vinblastine treatment are of major concern in the survivors and natural products like naringin may help to neutralize these effects. Therefore, a study was planned to evaluate the effect of naringin on the DNA damage induced by vinblastine in V79 cells. Vinblastine produced micronuclei formation in binucleate and second-division trinucleate V79 cells in Naringin + Vinblastine groups, whereas naringin pretreatment significantly reduced the vinblastine-induced micronuclei formation. Vinblastine is an antimitotic agent that inhibits tubulin formation and inhibits spindle formation required for mitosis.31 Vinblastine treatment was cytotoxic to mouse sperms.32 Vindesine, a semisynthetic derivative of vinblastine has been reported to increase the frequency of micronuclei in V79 cells dose-dependently.33 Vinblastine has been reported to induce clastogenic effect by producing micronuclei in the bone marrow cells of mice.34 Likewise, other antimitotic agent taxol also increased the micronuclei in V79 cells.30
The micronuclei formation was consistently detected in mononucleate cells in both the Naringin and Naringin + Vinblastine groups. The presence of micronuclei in mononucleate cells seems to be due to the escape of these cells from cytochalasin-B labeling or the failure of mononucleate cells to divide due to high genomic loss. Very few investigators have scored micronuclei in mononucleate cells after cytokinesis blockage of cells.35,36 The vinblastine also induced the formation of mononucleate, binucleate, and trinucleate cells with two and > two micronuclei indicating that it induced complex multiply sites of DNA damage and cells were unable to repair the complex DNA damage.37 The presence of micronuclei in trinucleate cells is due to nuclear division of binucleate cells and the presence of cytochalasin B did not allow the cell separation. Since vinblastine is a tubulin inhibitor the formation of micronuclei in V79 cells is due to spindle dysfunction, lagging chromosome during cell division, chromatid condensation, fragmentation of chromosomes like acentric fragments, DNA double strand breaks generated due to pre-mitotic DNA replication stress, and mis-repair of DNA breaks.38–40
Naringin significantly reduced the formation of vinblastine-induced micronuclei in mononucleate, binucleate, and trinucleate cells. Naringin also alleviated the spontaneous micronuclei formation in binucleate cells indicating that it protected the DNA from daily wear and tear. There are no reports on the reduction of vinblastine-induced DNA damage by naringin. However, naringin protected V79 cells against bleomycin-induced micronuclei formation and molecular DNA damage.21 The exact mechanism of action of naringin in reducing the vinblastine-induced DNA damage is not known. However, free radical formation and increased oxidative stress play a pivotal role in inducing DNA damage and reduction of micronuclei by naringin is due to its ability to neutralize free radical formation and reduce oxidative stress.12 Vinblastine induces 8-hydroxy-2-deoxyguanosine (8-OHdG) DNA adducts in human lymphocytes and naringin reduces the formation of these adducts due to its ability to elevate catalase, glutathione peroxidase, glutathione-s-transferase, and superoxide dismutase and reduced glutathione (GSH) in vivo.15,41–44 This increase in antioxidants by nariginin is due to its ability to upregulate Nrf2.45–47 The reduction in micronuclei in the present study is due to the activation of DNA repair pathways by naringin that may seal the DNA SSBs and DNA DSBs by suppressing the overactivation of poly (ADP-ribose) polymerases (PARPs) as their overactivation triggers genomic instability.15,48,49 The overactivation of PARP triggers nuclear translocation of NF-κB and its transcriptional activation leads to DNA damage and inhibition of NF-κB and COX-2 by naringin seems to protect against it.50–53 The cell culture techniques do not provide information on long-term genomic instability due to the use of cytochalasin-B which provides information on DNA damage after one cell division thereafter the DNA damage gets diluted due to cell multiplication or death of highly damaged cells.
Vinblastine treatment increased the frequency of mononucleate, binucleate, and trinucleate V79 cells bearing one two, and more than two micronuclei whereas naringin pretreatment significantly reduced the mononucleate, binucleate, and trinucleate cells with micronuclei. This reduction in DNA damage in the form of micronuclei may be due to free radical scavenging, an increase in antioxidant status, and Nrf2 followed by a decline in PARP, NF-κB, and COX-II activation. The naringin treatment also efficiently reduced spontaneous DNA damage in V79 cells. The present study demonstrates that the grapefruit bioflavonoid naringin can reduce genotoxicity in V79 cells.
This work was supported by the Indian Council of Medical Research, New Delhi, India vide grant No. 58/8/2000~BMS.
There is no conflicts of interest.

©2023 Jagetia. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.