MOJ
eISSN: 2576-4519


Research Article Volume 7 Issue 1
1Zootecnista, Facultad de Ciencias Pecuarias, Universidad de Nariño, Colombia
2Zoot, Esp, M.Sc, Ph.D, Profesor Titular de Tiempo Completo, Universidad de Nariño, Colombia
3Zoot, M.Sc, Profesora hora catedra, Universidad de Nariño, Colombia
Correspondence: Henry Jurado-Gámez, Zoot, Esp, M.Sc, Ph.D, Director Grupo de Investigación PROBIOTEC-FORAPIS, Profesor Titular de Tiempo Completo, Programa de Zootecnia, Facultad de Ciencias Pecuarias, Universidad de Nariño, Pasto, Colombia, Tel +573128333159
Received: October 25, 2023 | Published: November 9, 2023
Citation: Jurado-Gámez H, Ceron-Cordoba JF, Dávila-Solarte AP. Viability of microencapsulated Lactobacillus plantarum microencapsulated under simulated gastrointestinal conditions and its probiotic effect on Campylobacter jejuni. MOJ App Bio Biomech. 2023;7(1):198-203. DOI: 10.15406/mojabb.2023.07.00195
Probiotics, microorganisms that can benefit animal or human hosts, face application challenges due to environmental factors that reduce their stability. However, techniques like microencapsulation improve survival rates. Additionally, probiotics possess antibacterial properties that can inhibit the growth of pathogenic bacteria accountable for foodborne illnesses. The aim of this study was to assess the viability of microencapsulated Lactobacillus plantarum under simulated gastrointestinal conditions and its potential probiotic impact on Campylobacter jejuni. Fermentation kinetics was evaluated in an MRS culture medium over 24 hours. The growth of L. plantarum at 37°C and 45°C was examined, as well as microencapsulation through spray drying. Additionally, exposure to simulated gastrointestinal conditions was analyzed, while inhibition tests of L. plantarum on C. jejuni were performed. Finally, exopolysaccharide production from L. plantarum was assessed. The study findings demonstrated the termination of the exponential growth phase after 15 hours, improved development of lactic bacteria at 37°C, microencapsulation parameters within acceptable limits, survival of the microencapsulated strain in in vitro gastrointestinal conditions exceeding 7x108 UFC, and significant inhibitory effects of L. plantarum on pathogenic bacteria. The viability of microencapsulated Lactobacillus plantarum, subjected to simulated gastrointestinal conditions, exceeded 7x108 CFU/mL and demonstrated a probiotic effect on Campylobacter jejuni.
Keywords: probiotic, prebiotic, Lactobacillus, public health, encapsulation
MB, Mitochondrial diseases; mtDNA, mitochondrial DNA; MRI, magnetic resonance imaging; GTCS, generalized tonic-clonic seizure; HSV, herpes simplex virus; ENMG, electroneuromyography
"Probiotic" is a term that refers to the ability to confer a potential benefit on intestinal, immune, and cardiovascular health.1 They are mainly used to treat digestive disorders, gastrointestinal diseases, skin, mouth, urinary tract, and respiratory diseases.1,2
In general, Lactic Acid Bacteria (LAB) are considered probiotics, are widely distributed in nature and have been isolated from a variety of nutrient-rich habitats such as food, feed, vegetables, soils, fermented foods, animal and human intestinal mucosa.1 LAB are widely used in biotechnology, food, therapeutic products, control of pathogenic bacteria, antifungal agents, bacteriocin production, nutraceuticals, and as starter cultures in fermented foods.1,3,4 The range of uses is thanks to the versatile metabolism and the ability to synthesize a variety of metabolites such as lactic acid, organic acids, exopolysaccharides, bacteriocins, and biocins.3,5
The genus Lactobacillus is the most commonly utilized lactic acid microorganism, notably L. plantarum, which is found in diverse ecological niches and has significant commercial value in producing dairy products, pickles, sausages, sourdough, and other products.6 The potential probiotic effects of L. plamtarum are featured in numerous scientific studies.7,8 One parameter evaluated in probiotic characterizations is the survival ability in gastrointestinal conditions, including pH, acidity, enzymes, and temperature.1
In vivo gastrointestinal assays are challenging to perform in both humans and animals due to their expensive nature and ethical concerns. As a result, in vitro models have been developed to model food digestion.9 These models utilize uniform ratios of substrates such as food, drugs, and other compounds to enzymes, electrolytes, and pH for each stage of digestion.9,10
LAB probiotics face unfavorable conditions such as temperature, humidity, water activity, and pH. However, microencapsulation can mitigate these stressors by forming a protective layer around a bioactive substrate.11 This barrier is created from prebiotic matrices, which promote selective growth of bacteria in the intestines.12
Campylobacter, a bacterial genus, is a leading cause of acute bacterial enteritis.13 Campylobacter jejuni is responsible for 80% of cases of campylobacteriosis, which is characterized by fever, diarrhea, and abdominal colic. The main route of pathogenic microorganism spread is through cross-contamination of meat products, even after the refrigeration process.14,15 Moreover, C. jejuni strains from poultry farms have developed resistance to the antibiotics erythromycin, tetracycline, and ciprofloxacin.14
The aim of the study was to evaluate the viability of microencapsulated Lactobacillus plantarum ATCC 8014® under simulated gastrointestinal conditions and its probiotic effect on Campylobacter jejuni ATCC 33560®.
This research was conducted at the PROBIOTEC-FORAPIS research group laboratory, Teaching Laboratories block, Universidad de Nariño, Pasto Nariño. The strains Lactobacillus plantarum ATCC 8014® and Campylobacter jejuni ATCC 33560® were obtained from the MDM Científica supplier.
Fermentation kinetics: The fermentation kinetics were analyzed by measuring the growth of L. plantarum in the MRS culture medium. The L. plantarum inoculum was adjusted to the maximum McFarland scale and incubated at 37°C for 24 hours. Five samples were collected every three hours to measure the following kinetic variables: colony forming units (CFU) using spectrophotometry, sugar consumption (mg/L),16 protein production (mg/L),17 pH, and percentage of lactic acid.
Growth at different temperatures: The growth of L. plantarum was evaluated at two different temperatures (37°C and 45°C).18
Gas production and catalase activity: The gas production of L. plantarum was inoculated in MRS broth (37°C/24hours) enriched with 5% anhydrous glucose and placed in Durham hoods. The catalase activity of L. plantarum biomass was placed on a clean slide and was added small amount of hydrogen peroxide (30%). The formation of bubbles after the addition of hydrogen peroxide indicated a positive test for catalase activity.18
Microencapsulation - spray drying: The microencapsulation process was carried out with inlet temperature of 155°C and outlet temperature between 67-70°C, complete cycle of 3 hours and 25 minutes. 500mL of inoculum was prepared with addition of 15% w/v of microencapsulating material (37.5 g maltodextrin + 37.5 g inulin). The microencapsulated powder was packed in metalized zipper bags and stored at room temperature (17±2°C) for 32 days.
The physical and stability analysis of the microencapsulation on the microorganism was performed according to the following variables: viability (%), microencapsulation efficiency (%), humidity (%) (humidimetric balance), solubility (%), wettability (time) and water activity (aw),19 aW was evaluated for 32 days.
Exposure to simulated gastrointestinal conditions: 1g of microencapsulated powder was taken and added to 100 mL of distilled water. (Phase I) 25mL of phosphate buffer solution (0.1 M; pH 6) and 10mL of 0.2 M HCl were added and shaken for 5 min. (Phase II) The pH was adjusted to 2 using 1M HCl, and 1mL of pepsin solution in 0.2 M HCl (concentration of 25 mg/mL) was added and mixed well. The vessel was sealed and incubated at 40°C for 120 min. (Phase III) 10 mL of a buffered phosphate solution (0.2 M; pH 6.8) and 5mL of a 0.6M NaOH solution were added to maintain a stable pH 6.8 (NaOH 1M), 1mL of a 100 mg/mL pancreatin solution (porcine, grade VI, Sigma n. P-1750), vessels were sealed and incubated at 37°C for 300 min. 1mL of sample was removed from each phase for plate counting.9,11
Inhibition tests: The inhibition power of L. plantarum at 50µL, 80µL, and 110µL concentrations was evaluated against pathogens by different methods: 1) impregnated agar discs, 2) impregnated pads, 3) single-layer diffusion, and 4) double-layer diffusion. Positive criteria were halos equal to or greater than 2 mm.20
Exopolysaccharide (EPS) production: It was carried out at different temperatures and periods: 28±2ºC / 168 days, 35±2ºC / 48 hours, and 42±2ºC / 24 hours. EPS production was determined by the presence or absence of mucoid colonies at the base of the discs, then the presence of EPS was confirmed by mixing mucoid colonies in 2 ml of absolute alcohol.21,22
Fermentation kinetics: The variables evaluated in the fermentation kinetics are plotted in Figure 1, where the bacterial population in Ln (CFU/mL) is contrasted with sugar consumption, protein determination, pH, and % acidity.
The graphs with the data obtained in the fermentation kinetics allow establishing hour 15 as the end point of the exponential phase of bacterial growth with 23.25 Ln (1.3x1010 CFU/mL), sugar 8.47 mg/L, protein 4.91 mg/L, pH 4.18 and 1.21 % acidity. The ranges of the evaluated variables are described as follows: bacterial population 18.32 Ln (9x107 CFU/mL) to 21.79 Ln (2.9x109 CFU/mL) (Figure 1a), sugar 14.87 mg/L to 5.94 mg/L (Figure 1a), protein 9.74 mg/L to 2.84 mg/L (Figure 1b); percent acidity 0.37 % to 1.42 % (Figure 1c) and pH 6.45 to 3.68. The specific growth rate is 0.315 µmax h-1, cell duplication time 131.83 minutes, and generations per hour 0.46, with R2 = 0.9502.
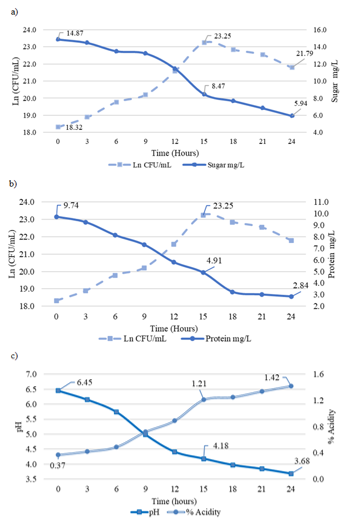
Figure 1 Variables evaluated in fermentation kinetics of L. plantarum in MRS culture medium:
a) Sugar consumption vs Ln (CFU/mL) L. plantarum,
b) Determination of proteins vs Ln (CFU/mL) L. plantarum,
c) Determination of pH and % acidity.
The reduction of carbohydrate and protein sources, tendency of pH to acidic levels, and increase of acidity percentage are related to the metabolism and products of LAB.23 They absorb carbohydrates as a source of energy and protein nitrogen for microbial wall formation and cell growth.24 The acidity of the culture medium increases due to the biosynthesis of organic acids (lactic acid) and the release of enzymes, biocins, and EPS. These by-products are known as the mechanisms that exert antimicrobial, antioxidant, and probiotic activities.25 LAB accelerates the active acidification of finished products and culture media and also lowers the pH to some extent (it is variable for each bacterial strain), which can inhibit the growth of undesirable bacteria.23
Each LAB has a different growth rate, and represents the rate at which each cell forms new individuals that depends on the environmental conditions and the availability of nutrients required by the microorganism,26 despite the existence of scientific research on lactic acid bacteria with fermentative kinetics data, the variability of the values confirm the specificity of the microbial growth curve for each probiotic.20
Growth at different temperatures: The results showed that the growth of L. plantarum was higher at 37°C temperature when compared to 45°C temperature (p<0.05); the growth values in Figure 2 are expressed as Ln 30.65 (2.05x1013UFC/mL) and 28.29 (1.93x1012UFC/mL) for 37°C and 45°C respectively. Figure 2 indicates significant statistical differences in the variable evaluated.
Optimal growth conditions optimize the lactic fermentation process, factors such as temperature must be controlled within the specific ranges for each microorganism.27 Good development of the microorganism is reflected in the action of the probiotic characteristics of LAB, the increase or decrease of the optimum temperature causes the inactivation of intracellular enzymes and the reduction of microbial activity.27,28
Gas production and catalase activity: Gas production and catalase activity were negatives for L. plantarum. The evaluation of gas production in LAB allows for identifying that there will be no intestinal gas accumulation in possible oral applications, and catalase activity is a proper indicator in the identification of LAB.29
Microencapsulation - spray drying: Water activity over time has an increasing behavior over time (Figure 3). With significant statistical differences on days 24 and 36 (p<0.05). The curve responds to a polynomial equation with R2 = 0.9832.
The aw and moisture are limiting factors that affect the stability of dehydrated foods, and characteristics such as molecular weight and adsorption/absorption properties of the wall materials have an impact on the characteristics of the final product.30 During the storage period, the aw of the microencapsulation remains between 0.2578 and 0.3088, a range lower than those established as critical for several dehydrated foods.31
The moisture content of the microencapsulate was 3.45% after 32 days of storage. Homayouni-Rad30 obtained moisture contents of L. casei, microencapsulated between 2.72% to 3.72%, in addition, several investigations mention that moisture contents lower than 4% increase the stability of the microencapsulated product during storage periods.20,30,32
The results of microencapsulated L. plantarum after 32 days of storage are described as follows: 93.2% efficiency, 91.37% viability, 3.45% moisture, 96.74% solubility and 83 sec wettability. Microencapsulation efficiency and viability refer to the CFU/mL available after the microencapsulation process, properties that depend directly on microencapsulation conditions (inlet and outlet temperature), equipment handling, encapsulant matrix composition and interaction with LAB.20,33 The evaluated variables and their behaviors are directly related to the wall materials used for microencapsulation. Maltodextrin has high solubility and provides low viscosity, in contrast, inulin has moderate solubility that complements the wall structure formed around the encapsulated substrate.10 The solubility of microencapsulation is a desirable characteristic in the food industry indicating high potential for use as a food ingredient.34
The gain or loss of moisture until reaching equilibrium with the relative humidity of the environment depends on the packaging systems, which allow giving stability to the stored substrate; sealable aluminum packaging has low water vapor permeability.35 The stability of the microencapsulated material is mainly reflected in the aW, humidity and growth of L. plantarum.
Structural characterization: Scanning Electron Microscopy (SEM) was performed after 32 days of storage. The microencapsulate shows slight agglomeration, with individual spherical, uniform microcapsules with diameters between 2.69µm - 5.72µm (Figure 4). Capsules with diameters ranging from 0.2µm to 5000µm are classified as microparticles.33 Different researchers report 2.58µm to 25.3µm in diameter in microencapsulated Lactobacillus with defined uniform structure.11,30 The formation of spherical and homogeneous microcapsules increases the stability of microencapsulated substrate and, the index of resistance against adverse conditions.11
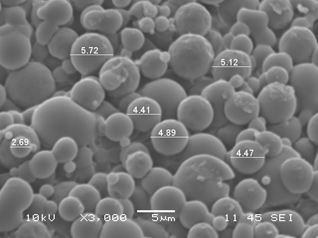
Figure 4 Scanning Electron Microscopy (SEM) of L. plantarum microencapsulated with inulin and maltodextrin by spray drying.
Measurements taken in ImageJ Processing and Analysis in Java (ImageJ) software. Source: Own elaboration.
Exposure to in-vitro gastrointestinal conditions: The final plate count was 2.6x108 CFU/mL (Figure 5), higher than the minimum recommended counts.12 Microencapsulation of probiotics improves product resistance to extreme factors such as temperature, humidity, storage, enzymes, and pH.11 One of the characteristics of probiotics is intrinsic resistance against gastrointestinal conditions. There are reports where the initial population is significantly reduced during gastric passage.36 Exposure of microencapsulated L. plantarum to gastrointestinal conditions indicates that the encapsulation process with inulin and maltodextrin protects the microorganism, improves bioavailability, and possibly releases at the site of interest.10,34
Inhibition test: The evaluation of Lactobacillus on C. jejuni indicates the inhibitory power of BAL on the pathogenic bacterium (Figure 6). The inhibition halos formed by agar discs with different concentrations of the probiotic are greater than 2 mm.20 The concentrations used showed that there is a relationship between inhibition halo and concentration (linear and directly proportional). The values obtained were 26.1mm, 33.5mm, and 42.6mm for the concentrations 50µm, 80µm and 110µm, respectively.
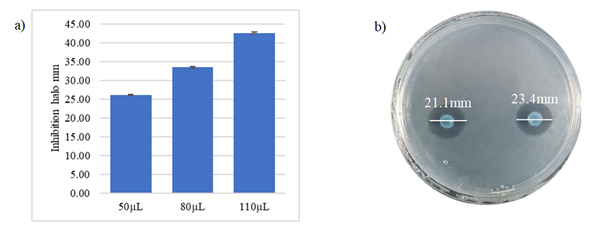
Figure 6 a) Halos of average inhibition assays of L. plantarum on C. jejuni by the agar method impregnated with L. plantarum
b) Agar with L. plantarum.
The study of the concentrations using the PADs method allowed observing that the lowest inhibition halos were found in the supernatant of L. plantarum at ambient conditions and without filter, and the highest halos were seen at temperatures of 80°c and filtered supernatant. The monolayer diffusion method demonstrated similar halos for the evaluations. Double-layer diffusion inhibition analyses showed no differences. All halos formed by the different inhibition methods, under different conditions and concentrations and that both biomass and supernatant of L. plantarum inhibit the growth of C. jejuni.
The microorganisms responsible for FBD (Foodborne Disease) correspond to the genera Salmonella, Campylobacter, and Shigella.37 Although these pathogenic bacteria have been reported to be resistant to antibiotics there is the possibility of controlling them with the use of LAB.38 Probiotics become an alternative system in preventive health and food safety. The bactericidal potential of LAB is given thanks to the metabolic formation of organic acids, acidification of the environment, cell adhesion, biofilm, and control of virulent genes (atpD, aguD).39 Probiotic strains have intrinsic properties of each species and present different mechanisms for inhibition and biomass growth.20
Exopolysaccharide production: After bacterial growth, EPS production is characterized by the formation of a viscous and filamentous layer.40 In the evaluated strain the production capacity was under different conditions of temperature and time. EPS products of BAL are Generally Recognized As Safe (GRAS) biopolymers, due to their antioxidant, antibacterial, antibiofilm, and antitumor properties.41 They have the property of improving sensory properties such as viscosity and texture, therefore, they are used in the food industry and contribute to the interaction with intestinal epithelial cells.42 Moreover, they have antioxidant properties and can be used as a natural antioxidant or food additive.43 In the same vein, Xu et al44 mentioned that EPS isolated from L. casei NA-2 inhibits biofilm formation and dispersion for B. cereus, S. aureus, S. typhimurium, and E. coli O157:H7.
The results obtained allow inferring: the end of the exponential phase of growth 15 hours with 1.3x1010UFC/mL, sugars 8.47mg/mL, protein 4.91mg/L, pH 4.18, % acidity 1.21. Growth is higher at 37°C.
Microencapsulation parameters (aW, humidity, particle diameter, viability, efficiency) within ranges that guarantee stability for 40 days of storage. Further investigation into the optimization of microencapsulation conditions is necessary to improve the viability and efficacy of L. plantarum during storage.
Bacterial growth after being subjected to gastrointestinal conditions of 2.6x108UFC/mL. It is recommended to conduct in vivo studies in animal models or clinical trials to confirm the efficacy of microencapsulated L. plantarum in a biological setting.
Inhibitory action of L. plantarum on C. jejuni and positive exopolysaccharide production at different temperatures. Explore the mechanisms behind the inhibition of C. jejuni by L. plantarum to enhance its probiotic potential and health benefits.
The evaluation of L. plantarum microencapsulated and subjected to different conditions presents a high viability. Results were obtained showing the inhibitory potential of C. jejuni. This study establishes the foundation for future research that may enhance the progress of functional foods and probiotic treatments.
Thanks to Vicerrectoría de Investigación e interacción Social (VISS), and PROBIOTEC-FORAPIS research group for the economic contribution and facilities to develop the research.
Research funded by Vicerrectoría de Investigación e interacción Social (VISS) - Universidad de Nariño, through Convocatoria de Investigación Docente 2021.
The authors declare that there are no conflicts of interest.

©2023 Jurado-Gámez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.